
Chemistry, 21.07.2019 11:00 agarcia24101993
The voltage generated by the zinc concentration cell described by the line notation zn(s) ∣∣ zn2+(aq,0.100 m) ∥∥ zn2+(aq,? m) ∣∣ zn(s) is 14.0 mv at 25 °c. calculate the concentration of the zn2+(aq) ion at the cathode.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:00
The pressure inside a hydrogen-filled container was 2.10 atm at 21 ? c. what would the pressure be if the container was heated to 92 ? c ?
Answers: 2

Chemistry, 22.06.2019 03:30
If a solution is considered basic, then a) the hydroxide ion and hydronium ion concentrations are equal. b) the hydroxide ion concentration is less than the hydronium ion concentration. c) the hydronium ion concentration is greater than the hydroxide ion concentration. d) the hydroxide ion concentration is greater than the hydronium ion concentration.
Answers: 1


Chemistry, 22.06.2019 12:30
Which element has the lowest electronegativity? calcium(ca) gallium(ga) selenium(se) bromine(br)
Answers: 1
You know the right answer?
The voltage generated by the zinc concentration cell described by the line notation zn(s) ∣∣ zn2+(aq...
Questions



Computers and Technology, 15.06.2021 04:40

Mathematics, 15.06.2021 04:40


Health, 15.06.2021 04:40



Mathematics, 15.06.2021 04:40

Biology, 15.06.2021 04:40

Mathematics, 15.06.2021 04:40




Mathematics, 15.06.2021 04:40


Physics, 15.06.2021 04:40

Mathematics, 15.06.2021 04:40


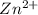 ion at cathode is 0.295 M
ion at cathode is 0.295 M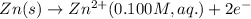
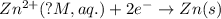
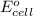 will be equal to zero.
will be equal to zero.![E_{cell}=E^o_{cell}-\frac{0.0592}{n}\log \frac{[Zn^{2+}]_{anode}}{[Zn^{2+}]_{cathode}}](/tpl/images/0115/3857/2e4c8.png)
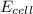 = 14.0 mV = 0.014 V (Conversion factor: 1 V = 1000 mV)
= 14.0 mV = 0.014 V (Conversion factor: 1 V = 1000 mV)![[Zn^{2+}]_{anode}](/tpl/images/0115/3857/a4cd7.png) = 0.100 M
= 0.100 M![[Zn^{2+}]_{cathode}](/tpl/images/0115/3857/54f88.png) = ? M
= ? M![0.014=0-\frac{0.0592}{2}\log \frac{0.100M}{[Zn^{2+}]_{cathode}}](/tpl/images/0115/3857/e07d0.png)
![[Zn^{2+}]_{cathode}=0.295M](/tpl/images/0115/3857/a683c.png)


