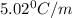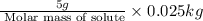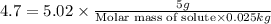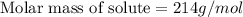Chemistry, 21.07.2019 10:30 Naysa150724
What is the molar mass of a solution of 5.00 g of a compound in 25.00 g of carbon tetrachloride (bp 76.8 °c; kb = 5.02 °c/m) that boils at 81.5 °c at 1 atm? (a) outline the steps necessary to answer the question?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 10:30
Find the number of grams of hcl needed to react completely with .50 moles of magnesium.
Answers: 1

Chemistry, 22.06.2019 16:00
Which factor is likely to impact the possible number of compounds ?
Answers: 1

Chemistry, 22.06.2019 16:30
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3

Chemistry, 23.06.2019 00:00
The empirical formula of a compound is ch2o and its mass is 120 amu/molecule, what is its formula?
Answers: 1
You know the right answer?
What is the molar mass of a solution of 5.00 g of a compound in 25.00 g of carbon tetrachloride (bp...
Questions

Mathematics, 15.07.2019 04:30

Biology, 15.07.2019 04:30




Business, 15.07.2019 04:30



Spanish, 15.07.2019 04:30


Mathematics, 15.07.2019 04:30


Mathematics, 15.07.2019 04:30

Mathematics, 15.07.2019 04:30


History, 15.07.2019 04:30

Biology, 15.07.2019 04:30



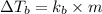
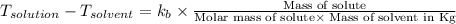
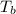 = change in boiling point =
= change in boiling point =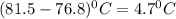
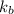 = boiling point constant =
= boiling point constant =