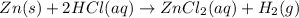Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 01:30
Idon't really understand this can you me and show your work.☺☺[ chemistry b] subject [ electron transfer in lonic bonds]grade( 12)
Answers: 1

Chemistry, 22.06.2019 04:40
In which environment would primary succession occur? a forest with a few remaining trees after a recent wildfire an area of exposed rock after a glacier melts away beach that is exposed to the air at low tide an abandoned baseball field in a small town
Answers: 1

Chemistry, 22.06.2019 11:00
Imagine that twenty i.u.’s of enzyme z were catalyzing the above reaction for one minute, under vmaxconditions, in a 3.00 ml assay volume. the assay is buffered with 20 mm phosphate buffer, ph 7.60. what will the ph be at the end of that one minute?
Answers: 2

Chemistry, 22.06.2019 12:30
Acontrol during an experiment. might change remains constant does not exist does change
Answers: 1
You know the right answer?
Given the reaction: zn(s) + 2hcl(aq) → zncl2(aq) + h2(g) the oxidation number of zn(s) increases be...
Questions






Mathematics, 16.04.2022 06:10

History, 16.04.2022 07:00

Mathematics, 16.04.2022 07:20



Mathematics, 16.04.2022 07:40




Business, 16.04.2022 08:30

English, 16.04.2022 08:30

Mathematics, 16.04.2022 08:50


Biology, 16.04.2022 09:10