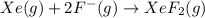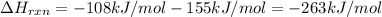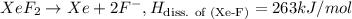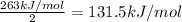Chemistry, 21.07.2019 07:30 Jessieileen
The enthalpy of formation of xef2(g) is –108 kj mol–1 and the bond dissociation enthalpy of the f–f bond is 155 kj mol–1 . what is the average bond dissociation enthalpy of a xe–f bond

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 12:10
|using the periodic tablewarm-upuse the periodic table in the tools bar to answer the following questions.what elemental classification does oxygen belongto? done
Answers: 3

Chemistry, 22.06.2019 18:30
How many moles of lead are in 1.50 x 10^12 atoms of lead? could you explain the answer as well and not just give it to me i am refreshing for finals and i need to know how to do it
Answers: 3

Chemistry, 22.06.2019 21:00
Use the measurements in the table to determine which unidentified metal has the highest density. metal volume mass a 10.5 cm3 122 g b 14.2 cm3 132 g c 16.1 cm3 115 g d 12.7 cm3 126 g
Answers: 2

Chemistry, 22.06.2019 22:30
Amedication is given at a dosage of 3.000 mg of medication per kg of body weight. if 0.1500 g of medication is given, then what was the patient's weight in pounds (lbs)? there are 453.59g in 1 lb.
Answers: 2
You know the right answer?
The enthalpy of formation of xef2(g) is –108 kj mol–1 and the bond dissociation enthalpy of the f–f...
Questions

Mathematics, 21.11.2019 14:31



Mathematics, 21.11.2019 14:31

Mathematics, 21.11.2019 14:31



English, 21.11.2019 14:31



Mathematics, 21.11.2019 14:31

Geography, 21.11.2019 14:31

Spanish, 21.11.2019 14:31


Mathematics, 21.11.2019 14:31




Health, 21.11.2019 14:31

Social Studies, 21.11.2019 14:31

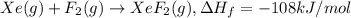 ..(1)
..(1)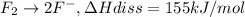 ..(2)
..(2)