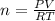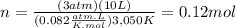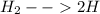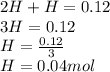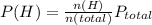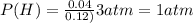Computers and Technology, 30.10.2019 06:31 jaylenmiller437
A10.0-l container is filled with 0.10 mol of h2(g) and heated to 3050 k. at that temperature, some of the h2(g) decomposes into h(g), and the total pressure is found to be 3.0 atm. find the partial pressure of the h(g) that has been formed. (keep in mind that there is a reaction taking place, so as h(g) is being formed, h2(g) is being consumed.)

Answers: 3
Another question on Computers and Technology

Computers and Technology, 23.06.2019 17:00
What are the 12 colors of the spectrum called?
Answers: 1

Computers and Technology, 23.06.2019 20:00
What multimedia system creates an immersive, real-life experience that the user can interact with?
Answers: 1

Computers and Technology, 24.06.2019 08:20
Evaluate the scenario below and indicate how to handle the matter appropriately. situation: michael received an e-mail from what he thought was his doctor’s office, requesting his social security number. since he had just been in to see his doctor last week, he replied to the e-mail with his social security number.
Answers: 2

Computers and Technology, 24.06.2019 13:00
If you add the following to the query grid in an access query, what is it called? salestaxamt: [salestaxrate]*[totalsale] formula calculated field total calculation
Answers: 2
You know the right answer?
A10.0-l container is filled with 0.10 mol of h2(g) and heated to 3050 k. at that temperature, some o...
Questions




History, 20.10.2019 00:50



History, 20.10.2019 00:50


History, 20.10.2019 00:50

Mathematics, 20.10.2019 00:50


Biology, 20.10.2019 00:50


History, 20.10.2019 00:50

Chemistry, 20.10.2019 00:50

English, 20.10.2019 00:50


History, 20.10.2019 00:50

Mathematics, 20.10.2019 00:50

Mathematics, 20.10.2019 00:50