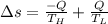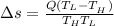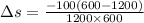Engineering, 13.07.2019 04:30 ghernadez
Heat in the amount of 100 kj is transferred directly from a hot reservoir at 1200 k to a cold reservoir at 600 k. calculate the entropy change of the two reservoirs and determine if the increase of entropy principle is satisfied.

Answers: 1
Another question on Engineering

Engineering, 04.07.2019 18:10
Abrake has a normal braking torque of 2.8 kip in and heat-dissipating cast-iron surfaces whose mass is 40 lbm. suppose a load is brought to rest in 8.0 s from an initial angular speed of 1600 rev/min using the normal braking torque; estimate the temperature rise of the heat dissipating surfaces.
Answers: 3

Engineering, 04.07.2019 18:10
The higher the astm grain size number, the finer the gran is. a)-true b)-false
Answers: 2

Engineering, 04.07.2019 18:10
Atmospheric air has a temperature (dry bulb) of 80° f and a wet bulb temperature of 60° f when the barometric pressure is 14.696 psia. determine the specific humidity, grains/lb dry air. a. 11.4 c. 55.8 d. 22.5 b. 44.1
Answers: 1

Engineering, 04.07.2019 18:10
Which of the following controllers anticipates the future from the slope of errors over time? a)-proportional b)-on/off c)-integral d)-derivative.
Answers: 2
You know the right answer?
Heat in the amount of 100 kj is transferred directly from a hot reservoir at 1200 k to a cold reserv...
Questions

Mathematics, 17.07.2020 05:01

Mathematics, 17.07.2020 05:01


English, 17.07.2020 05:01





Medicine, 17.07.2020 05:01




History, 17.07.2020 05:01

Mathematics, 17.07.2020 05:01





Mathematics, 17.07.2020 05:01

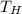 = 1200 K
= 1200 K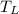 = 600 K
= 600 K