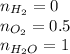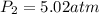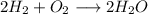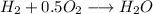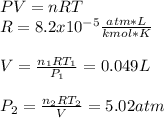Engineering, 02.09.2019 16:30 ineedhelp368
Amixture of 1 kmol of hydrogen (h2) and n kmol of oxygen (o2), initially at 25°c and 1 atm, burns completely in a closed, rigid, insulated container. the container finally holds a mixture of water vapor and o2 at 2000 k. the ideal gas model applies to each mixture and there is no change in kinetic or potential energy between the initial and final states. determine: (a) the value of n. (b) the final pressure, in atm.

Answers: 3
Another question on Engineering

Engineering, 04.07.2019 18:10
Coiled springs ought to be very strong and stiff. si3n4 is a strong, stiff material. would you select this material for a spring? explain.
Answers: 2

Engineering, 04.07.2019 18:10
Determine whether or not it is possible to compress air adiabatically from k to 140 kpa and 400 k. what is the entropy change during this process?
Answers: 3

Engineering, 04.07.2019 19:10
The sum of the normal stresses does not change as the stress state rotates through an angle. a)-trune b)- false
Answers: 2

Engineering, 04.07.2019 19:10
Apressure vessel with an r/t 20 cannot be treated as thin walled vessel. a)-trune b)- false
Answers: 3
You know the right answer?
Amixture of 1 kmol of hydrogen (h2) and n kmol of oxygen (o2), initially at 25°c and 1 atm, burns co...
Questions


Law, 06.02.2021 22:40

Mathematics, 06.02.2021 22:40




Physics, 06.02.2021 22:40




Mathematics, 06.02.2021 22:40


Social Studies, 06.02.2021 22:40

Mathematics, 06.02.2021 22:40


Mathematics, 06.02.2021 22:50


Chemistry, 06.02.2021 22:50