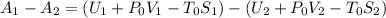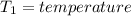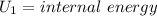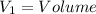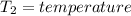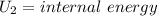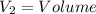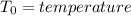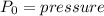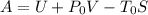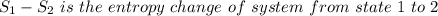Engineering, 02.09.2019 19:20 hgdthbgjnb5604
Aclosed thermodynamic system undergoes a process that takes it from state 1 to state 2. a) write the equation that describes the change in total availability (maximum energy available to do useful work). b) describe the meaning of each term in the equation.

Answers: 1
Another question on Engineering

Engineering, 03.07.2019 15:10
Apiston-cylinder with a volume of 0.25 m3 holds 1 kg of air (r 0.287 k/kgk) at a temperature of 100 c. heat transfer to the cylinder causes an isothermal expansion of the piston until the volume triples. how much heat is added to the piston-cylinder?
Answers: 3

Engineering, 04.07.2019 18:10
Steel is coated with a thin layer of ceramic to protect against corrosion. what do you expect to happen to the coating when the temperature of the steel is increased significantly? explain.
Answers: 1

Engineering, 04.07.2019 18:10
Water at 55c flows across a flat plate whose surface temperature is held constant at 95c. if the temperature gradient at the plate's surface for a given value of x is 18 c/mm, find a) local heat transfer coefficient. b) heat flux
Answers: 3

Engineering, 04.07.2019 18:10
Which of the following controllers anticipates the future from the slope of errors over time? a)-proportional b)-on/off c)-integral d)-derivative.
Answers: 2
You know the right answer?
Aclosed thermodynamic system undergoes a process that takes it from state 1 to state 2. a) write the...
Questions

Business, 31.05.2020 08:59







Biology, 31.05.2020 08:59





Mathematics, 31.05.2020 08:59

Mathematics, 31.05.2020 09:57

Biology, 31.05.2020 09:57

Mathematics, 31.05.2020 09:57

History, 31.05.2020 09:57