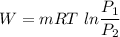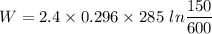Engineering, 13.09.2019 23:10 acebeann
2.4 kg of nitrogen at an initial state of 285k and 150 kpa is compressed slowly in an isothermal process to a final pressure of 600 kpa. determine the work.

Answers: 3
Another question on Engineering

Engineering, 04.07.2019 18:10
The temperature of air decreases as it is compressed by an adiabatic compressor. a)- true b)- false
Answers: 2

Engineering, 04.07.2019 18:10
Consider a large isothermal enclosure that is maintained at a uniform temperature of 2000 k. calculate the emissive power of the radiation that emerges from a small aperture on the enclosure surface. what is the wavelength ? , below which 10% of the emission is concentrated? what is the wavelength ? 2 above which 10% of the emission is concentrated? determine the wavelength at which maximum spectral emissive power occurs. what is the irradiation incident on a small object placed inside the enclosure?
Answers: 2

Engineering, 04.07.2019 18:10
An air compression refrigeration system is to have an air pressure of 100 psia in the brine tank and an allowable air temperature increase of 60°f for standard vapor compression cycle temperatures of 77 f entering the expansion cylinder and 14 f entering the compression cylinder, calculate the coefficient of performance a. 2.5 b 3.3 c. 4.0 d. 5.0
Answers: 3

Engineering, 04.07.2019 19:10
10 kg of co2 is initially contained at 400 kpa and 300 k. the gas constant for carbon dioxide is 189 j/lkg k) and has a specific heat ratio, k, of 1.289. isentropic expansion then occurs until the pressure is 200 kpa. a) determine the initial volume of co2 in m. b) determine the final temperature in k. c) determine the work done by the system during the expansion kl.
Answers: 2
You know the right answer?
2.4 kg of nitrogen at an initial state of 285k and 150 kpa is compressed slowly in an isothermal pro...
Questions

Mathematics, 31.03.2020 05:31



Mathematics, 31.03.2020 05:31


History, 31.03.2020 05:31

Chemistry, 31.03.2020 05:31





Mathematics, 31.03.2020 05:32


Mathematics, 31.03.2020 05:33


English, 31.03.2020 05:33

Mathematics, 31.03.2020 05:34


Mathematics, 31.03.2020 05:34

Mathematics, 31.03.2020 05:34