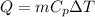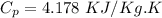Engineering, 13.09.2019 23:10 pandagorwar
Given the latent heat of fusion (melting) and the latent heat of vaporisation for water are δhs = 333.2 kj/kg and δhv = 2257 kj/kg, respectively. use these values to estimate the total energy required to melt 100 kg of ice at 0 °c and boil off 40 kg of water at 100 °c. a) 239,028 kj b) 95,250 kj c) 185,500 kj d) 362,628 kj e) 123,600 kj

Answers: 2
Another question on Engineering

Engineering, 04.07.2019 18:10
Steel is coated with a thin layer of ceramic to protect against corrosion. what do you expect to happen to the coating when the temperature of the steel is increased significantly? explain.
Answers: 1

Engineering, 04.07.2019 18:10
Atmospheric air has a temperature (dry bulb) of 80° f and a wet bulb temperature of 60° f when the barometric pressure is 14.696 psia. determine the specific humidity, grains/lb dry air. a. 11.4 c. 55.8 d. 22.5 b. 44.1
Answers: 1

Engineering, 04.07.2019 18:10
Journeyman training is usually related (clo2) a)-to specific tasks b)-to cost analysis of maintenance task c)-to control process to ensure quality d)-to installation of machinery
Answers: 2

Engineering, 04.07.2019 19:10
What is the chief metrological difference between measuring with a microscope and with an electronic comparator? a. the microscope is limited to small workpieces.a. the microscope is limited to small workpieces. c. the comparator can only examine one point on the workpiece. d. the microscope carries its own standard.
Answers: 1
You know the right answer?
Given the latent heat of fusion (melting) and the latent heat of vaporisation for water are δhs = 33...
Questions



Biology, 15.04.2020 16:12





Mathematics, 15.04.2020 16:12

English, 15.04.2020 16:12




Mathematics, 15.04.2020 16:13