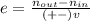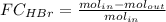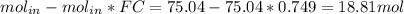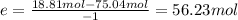Engineering, 16.10.2019 05:00 jnsoccerboy3121
The reaction between ethylene and hydrogen bromide to form ethyl bromide is carried out in a continuous reactor. the product stream is analyzed and found to contain 51.7 mole% c2h5br and 17.3% hbr. the feed to the reactor contains only ethylene and hydrogen bromide. calculate the fractional conversion of the limiting reactant and the percentage by which the other reactant is in excess. if the molar flow rate of the feed stream is 165mol/s, what is the extent of reaction?

Answers: 2
Another question on Engineering

Engineering, 03.07.2019 14:10
When at a point two solid phase changes to one solid phase on cooling then it is known as a) eutectoid point b) eutectic point c) peritectic point d) peritectoid point
Answers: 3

Engineering, 04.07.2019 12:10
On a average work day more than work place firs are reorted
Answers: 1

Engineering, 04.07.2019 18:10
Apump is used to circulate hot water in a home heating system. water enters the well-insulated pump operating at steady state at a rate of 0.42 gal/min. the inlet pressure and temperature are 14.7 lbf/in.2, and 180°f, respectively; at the exit the pressure is 60 lbf/in.2 the pump requires 1/15 hp of power input. water can be modeled as an incompressible substance with constant density of 60.58 lb/ft3 and constant specific heat of 1 btu/lb or. neglecting kinetic and potential energy effects, determine the temperature change, in °r, as the water flows through the pump.
Answers: 1

Engineering, 04.07.2019 18:10
Atmospheric air has a temperature (dry bulb) of 80° f and a wet bulb temperature of 60° f when the barometric pressure is 14.696 psia. determine the specific humidity, grains/lb dry air. a. 11.4 c. 55.8 d. 22.5 b. 44.1
Answers: 1
You know the right answer?
The reaction between ethylene and hydrogen bromide to form ethyl bromide is carried out in a continu...
Questions

Mathematics, 19.10.2019 17:10

Mathematics, 19.10.2019 17:10

Health, 19.10.2019 17:10


Spanish, 19.10.2019 17:10






Mathematics, 19.10.2019 17:10


Social Studies, 19.10.2019 17:20

Mathematics, 19.10.2019 17:20

Mathematics, 19.10.2019 17:20



Mathematics, 19.10.2019 17:20


Mathematics, 19.10.2019 17:20


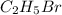 = 51.7%
= 51.7%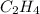 = 100% - 69% = 31%
= 100% - 69% = 31%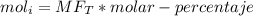
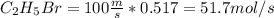
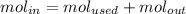
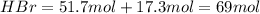
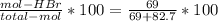 = 45.4%
= 45.4%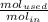
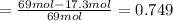
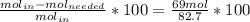 =16.56%
=16.56%