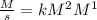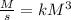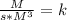Engineering, 24.10.2019 05:00 MZ2017
No reacts with br2 in the gas phase according to the following chemical equation: 2no(g) +br2(g)2nobr(g) it is observed that, when the concentration of br2 is reduced to 1/3 of its initial value, the rate of the reaction is also reduced to 1/3 of its initial value. when the concentration of no is multiplied by 3.69, the rate of the reaction increases by a factor of 13.6. (a) write the rate expression for this reaction, and give the units of the rate constant k, assuming concentration is expressed as mol l-1 and time is in second

Answers: 3
Another question on Engineering

Engineering, 03.07.2019 14:10
The y form of iron is known as: a) ferrite b) cementite c) perlite d) austenite
Answers: 3

Engineering, 03.07.2019 15:10
Heat is added to a piston-cylinder device filled with 2 kg of air to raise its temperature 400 c from an initial temperature of t1 27 cand pressure of pi 1 mpa. the process is isobaric process. find a)-the final pressure p2 b)-the heat transfer to the air.
Answers: 1

Engineering, 04.07.2019 16:10
The force on a cutting tool are 2600n vertically downward and 2100 horizontal. determine the resultant force acting on the tool and the angle at which it acts.
Answers: 1

Engineering, 04.07.2019 18:10
If a particle moves along a path such that r : (3 sin t) m and ? : 2t rad, where t is in seconds. what is the particle's acceleration in m/s in 4 seconds? a)- 16.43 b)- 16.29 c)- 15.21 d)- 13.79
Answers: 1
You know the right answer?
No reacts with br2 in the gas phase according to the following chemical equation: 2no(g) +br2(g)2no...
Questions

Biology, 21.11.2019 20:31


















History, 21.11.2019 20:31

![rate=r=k[NO]^{2} [Br_{2}]^{1}](/tpl/images/0344/0256/53abd.png)
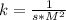
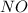 and
and 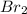 ):
):![[Br_{2} ]](/tpl/images/0344/0256/7c30c.png) ) is reduced to 1/3 of its initial value, the rate of the reaction is also reduced to 1/3 of its initial value, it means:
) is reduced to 1/3 of its initial value, the rate of the reaction is also reduced to 1/3 of its initial value, it means:![[Br_{2} ]=1/3](/tpl/images/0344/0256/095d6.png) then
then 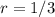
![[NO]](/tpl/images/0344/0256/5d819.png) ) is multiplied by 3.69, the rate of the reaction increases by a factor of 13.6. In this case, to know the ratio could be advisable divide the rate of the reaction (13.6) over the factor whereby was multiplied the concentration (3.69), as follows:
) is multiplied by 3.69, the rate of the reaction increases by a factor of 13.6. In this case, to know the ratio could be advisable divide the rate of the reaction (13.6) over the factor whereby was multiplied the concentration (3.69), as follows: 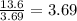
![r=[NO]^{2} =3.68^{2} =13.6](/tpl/images/0344/0256/fcec5.png)
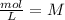 and time is in second, to find the units of k we need to solve an equation with units and with supporting of the rate equation previously obtained, as follows:
and time is in second, to find the units of k we need to solve an equation with units and with supporting of the rate equation previously obtained, as follows: ![r=k[NO]^{2} [Br_{2}]^{1}](/tpl/images/0344/0256/b261f.png)
![[r]=[\frac{M}{s}]](/tpl/images/0344/0256/01dc2.png)
![[[NO]]=[M]](/tpl/images/0344/0256/fc34d.png)
![[ [Br_{2}]]=[M]](/tpl/images/0344/0256/ba60f.png)