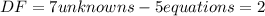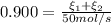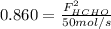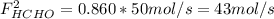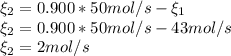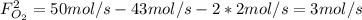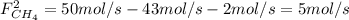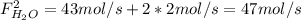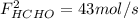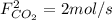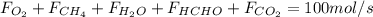Engineering, 15.11.2019 22:31 mkidgellmas1284
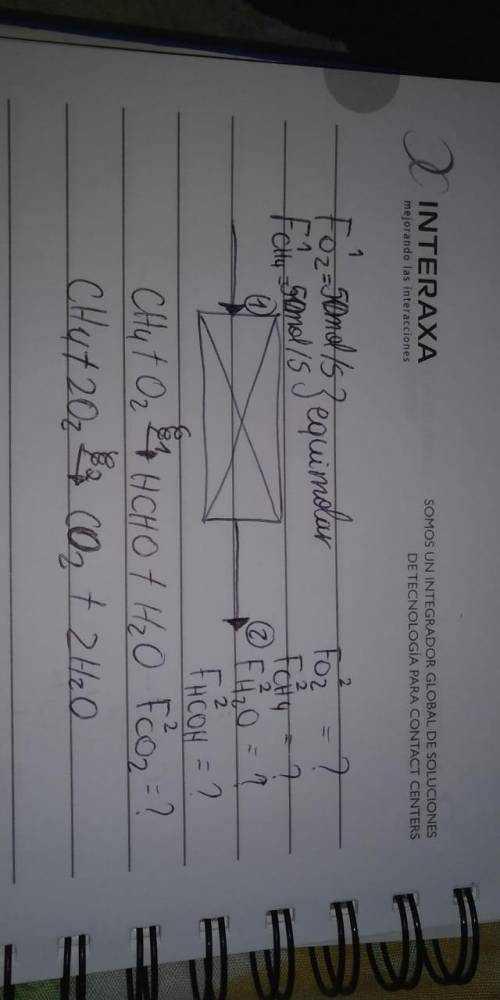
Methane and oxygen react in the presence of a catalyst to form formaldehyde. in a parallel reaction, methane is oxidized to carbon dioxide and water.
ch4 + o2 → hcho + h2o
ch4 + 2o2 → co2 + 2h2o
the feed to the reactor contains equimolar amounts of methane and oxygen. assume a basis of 100.0 mol feed/s.
a. how many degrees of freedom remain for the overall process?
b. the fractional conversion of methane is 0.900 and the fractional yield of formaldehyde is 0.860. what is the composition of the output stream?

Answers: 3
Another question on Engineering

Engineering, 03.07.2019 15:10
If you were designing a bumper for a car, would you prefer it to exhibit elastic or plastic deformation? why? consider the functions of a bumper in both a minor "fender-bender" and a major collision.
Answers: 1

Engineering, 04.07.2019 18:10
Abrake has a normal braking torque of 2.8 kip in and heat-dissipating cast-iron surfaces whose mass is 40 lbm. suppose a load is brought to rest in 8.0 s from an initial angular speed of 1600 rev/min using the normal braking torque; estimate the temperature rise of the heat dissipating surfaces.
Answers: 3

Engineering, 04.07.2019 18:10
The flow rate of air through a through a pipe is 0.02 m5/s. a pitot static tube is placed in the flow. the radius of the pitot static tube is 1 mm. assuming the flow to be steady and the air to be at 300k, calculate the difference in total and static pressure if the diameter of the pipe is: (a) d 0.1 m d 0.05 m (c) d 0.01 m
Answers: 2

Engineering, 04.07.2019 18:10
Courses that are developed by subject matter experts, internal or extemal to the college or university. these programs are marketed by the school (clo2) marks a)-vocational schools b)-vendor training c)-colleges & universities d)-continuing education programs
Answers: 2
You know the right answer?
Methane and oxygen react in the presence of a catalyst to form formaldehyde. in a parallel reaction,...
Questions


Mathematics, 05.07.2019 12:00

Mathematics, 05.07.2019 12:00


Health, 05.07.2019 12:00

Mathematics, 05.07.2019 12:00



English, 05.07.2019 12:00

Mathematics, 05.07.2019 12:00

Advanced Placement (AP), 05.07.2019 12:00

Mathematics, 05.07.2019 12:00

French, 05.07.2019 12:00

Mathematics, 05.07.2019 12:00

Mathematics, 05.07.2019 12:00

History, 05.07.2019 12:00

Health, 05.07.2019 12:00



Chemistry, 05.07.2019 12:00

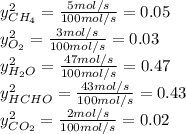
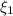 and
and 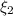 : extent of the reactions (2).
: extent of the reactions (2).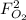 ,
, 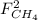 ,
, 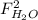 ,
, 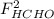 and
and 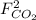 : Molar flows at the second stream (5).
: Molar flows at the second stream (5).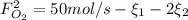 : oxygen mole balance.
: oxygen mole balance.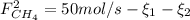 : methane mole balance.
: methane mole balance.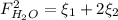 : water mole balance.
: water mole balance.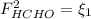 : formaldehyde mole balance.
: formaldehyde mole balance.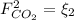 : carbon dioxide mole balance.
: carbon dioxide mole balance.