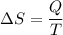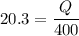Engineering, 06.12.2019 02:31 leahglover19
Agas within a piston–cylinder assembly undergoes an isothermal process at 400 k during which the change in entropy is 20.3 kj/k. assuming the ideal gas model for the gas and negligible kinetic and potential energy effects, evaluate the work, in kj.

Answers: 2
Another question on Engineering

Engineering, 03.07.2019 14:10
When at a point two solid phase changes to one solid phase on cooling then it is known as a) eutectoid point b) eutectic point c) peritectic point d) peritectoid point
Answers: 3

Engineering, 03.07.2019 15:10
If you were designing a bumper for a car, would you prefer it to exhibit elastic or plastic deformation? why? consider the functions of a bumper in both a minor "fender-bender" and a major collision.
Answers: 1

Engineering, 04.07.2019 18:10
Refrigerant 134a enters an insulated compressor operating at steady state as saturated vapor at -26°c with a volumetric flow rate of 0.18 m3/s. refrigerant exits at 9 bar, 70°c. changes in kinetic and potential energy from inlet to exit can be ignored. determine the volumetric flow rate at the exit, in m3/s, and the compressor power, in kw.
Answers: 1

Engineering, 04.07.2019 18:10
Thermal stresses are developed in a metal when its a) initial temperature is changed b) final temperature is changed c) density is changed d) thermal deformation is prevented e) expansion is prevented f) contraction is prevented
Answers: 2
You know the right answer?
Agas within a piston–cylinder assembly undergoes an isothermal process at 400 k during which the cha...
Questions

History, 29.01.2021 23:10

Mathematics, 29.01.2021 23:10

Mathematics, 29.01.2021 23:10


Geography, 29.01.2021 23:10


Mathematics, 29.01.2021 23:10


History, 29.01.2021 23:10

Mathematics, 29.01.2021 23:10

Mathematics, 29.01.2021 23:10


Geography, 29.01.2021 23:10

Spanish, 29.01.2021 23:10

Mathematics, 29.01.2021 23:10


Mathematics, 29.01.2021 23:10

Mathematics, 29.01.2021 23:10


Mathematics, 29.01.2021 23:10