Engineering, 11.02.2020 05:32 thesavagefatima
A copper block of volume 1 L is heat treated at 500°C and now cooled by immersion in a 200 L light oil bath initially at 20 °C. Assuming no heat transfer with the surroundings, determine the final equilibrium temperature of the copper-oil system assuming the specific heat capacities of both are constant.

Answers: 1
Another question on Engineering

Engineering, 04.07.2019 18:10
Water in a partially filled large tank is to be supplied to the roof top, which is 8 m above the water level in the tank, through a 2.2-cm-internal-diameter pipe by maintaining a constant air pressure of 300 kpa (gage) in the tank. if the head loss in the piping is 2 m of water, determine the discharge rate of the supply of water to the roof top in liters per second.
Answers: 3

Engineering, 04.07.2019 18:20
Have a greater impact on maintenance productivity than any other support group. (clo5) a)-the top management b)-inventory and purchasing c)-sub-contracting d)-cmms
Answers: 2

Engineering, 04.07.2019 18:20
Modern high speed trains do not have perpendicular expansion gaps where rails are joined end-to-end any more they are mostly welded together but what might happen if there was a spell of particularly hot weather that causes inspection of the tracks?
Answers: 1

Engineering, 04.07.2019 19:10
What is the main objective of using reheat rankine cycle?
Answers: 3
You know the right answer?
A copper block of volume 1 L is heat treated at 500°C and now cooled by immersion in a 200 L light o...
Questions

History, 11.05.2021 03:50


Mathematics, 11.05.2021 03:50




Mathematics, 11.05.2021 03:50








Chemistry, 11.05.2021 03:50

Mathematics, 11.05.2021 03:50




Mathematics, 11.05.2021 03:50

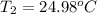
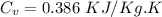
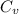 =
=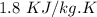
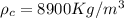
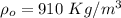
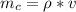 =
=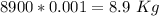
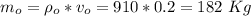
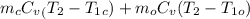 =0
=0