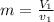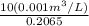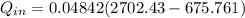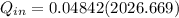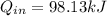Engineering, 15.02.2020 05:24 asyamelissa01
A rigid 14-L vessel initially contains a mixture of liquid water and vapor at 100°C with 12.3 percent quality. The mixture is then heated until its temperature is 180°C. The final state is superheated water and the internal energy at this state should be obtained by interpolation. Calculate the heat transfer required for this process. Use data from the steam tables.

Answers: 1
Another question on Engineering

Engineering, 04.07.2019 18:10
Which of the following refers to refers to how well the control system responds to sudden changes in the system. a)-transient regulation b)- distributed regulation c)-constant regulation d)-steady-state regulation
Answers: 1

Engineering, 04.07.2019 18:20
Agas mixture consists of 8 kmol of h2 and 2 kmol of n2. determine the mass of each gas and the apparent gas constant of the mixture.
Answers: 3

Engineering, 04.07.2019 18:20
Acertain flow of air (at stp) has a velocity distribution given by v i (in ft/s). if this flow is going through a 4 ft square area in the yz-plane (centered at the origin), what is the mass flow rate (in lbm/s)?
Answers: 2

Engineering, 04.07.2019 19:10
Apressure vessel with an r/t 20 cannot be treated as thin walled vessel. a)-trune b)- false
Answers: 3
You know the right answer?
A rigid 14-L vessel initially contains a mixture of liquid water and vapor at 100°C with 12.3 percen...
Questions

Biology, 04.05.2021 17:40






Biology, 04.05.2021 17:40

Mathematics, 04.05.2021 17:40


Mathematics, 04.05.2021 17:40



Mathematics, 04.05.2021 17:40


Chemistry, 04.05.2021 17:40

Chemistry, 04.05.2021 17:40



Mathematics, 04.05.2021 17:50

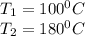
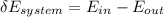
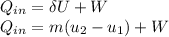
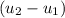 = corresponding change in the internal energy at state point 2 and 1
= corresponding change in the internal energy at state point 2 and 1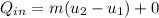
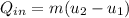
 , the given quality of mixture of liquid water and vapor
, the given quality of mixture of liquid water and vapor 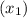 = 123% = 0.123
= 123% = 0.123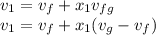
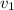 = specific volume at state 1
= specific volume at state 1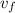 = specific volume of the liquid
= specific volume of the liquid 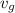 = specific volume of the liquid vapor mixture
= specific volume of the liquid vapor mixture 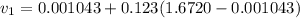
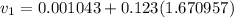
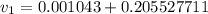
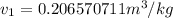
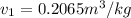
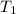 = 100°C and
= 100°C and 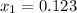 ;
;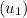 at the state 1
at the state 1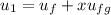
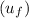 = 419.06 kJ/kg
= 419.06 kJ/kg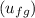 = 2087.0 kJ/kg
= 2087.0 kJ/kg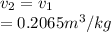
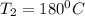 from the data in the steam tables
from the data in the steam tables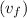 = 0.00113 m³/kg
= 0.00113 m³/kg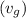 = 0.19384 m³/kg
= 0.19384 m³/kg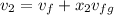
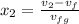
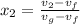
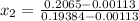
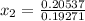
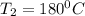
 = 1820.88 kJ/kg
= 1820.88 kJ/kg
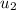 ≅ 2702.43 kJ/kg
≅ 2702.43 kJ/kg