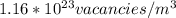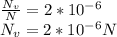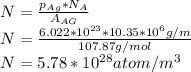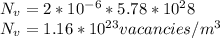Engineering, 07.03.2020 02:23 supermimi8078
The equilibrium fraction of lattice sites that are vacant in silver (Ag) at 700°C is 2 × 10-6. Calculate the number of vacancies (per meter cubed) at 700°C. Assume a density of 10.35 g/cm3 for Ag, and note that AAg = 107.87 g/mol.

Answers: 1
Another question on Engineering

Engineering, 04.07.2019 18:10
Aflywheel accelerates for 5 seconds at 2 rad/s2 from a speed of 20 rpm. determine the total number of revolutions of the flywheel during the period of its acceleration. a.5.65 b.8.43 c. 723 d.6.86
Answers: 2

Engineering, 04.07.2019 18:10
The higher the astm grain size number, the finer the gran is. a)-true b)-false
Answers: 2

Engineering, 04.07.2019 18:20
The characteristic roots of a dynamic system are: 1.7920 1.8160 i, -1.7920 1.8160 i, -0.4160 what is the order of this system? what are the settling time and damping ratio of the system?
Answers: 3

Engineering, 04.07.2019 19:10
With increases in magnification, which of the following occur? a. the field of view decreases. b. the ambient illumination decreases. c. the larger parts can be measured. d. the eyepiece must be raised.
Answers: 1
You know the right answer?
The equilibrium fraction of lattice sites that are vacant in silver (Ag) at 700°C is 2 × 10-6. Calcu...
Questions


Mathematics, 05.05.2020 04:11

Physics, 05.05.2020 04:11

Health, 05.05.2020 04:11


English, 05.05.2020 04:11




Mathematics, 05.05.2020 04:11

SAT, 05.05.2020 04:11




Mathematics, 05.05.2020 04:11




Mathematics, 05.05.2020 04:11