
Engineering, 24.03.2020 04:29 kris22elizondop9v1bb
The purification of hydrogen gas is possible by diffusion through a thin palladium sheet. Calculate the number of kilograms of hydrogen that pass per hour (in kg/h) through a 3.1-mm thick sheet of palladium having an area of 0.25 m2 at 500°C. Assume a diffusion coefficient of 6.0 x 10-8 m2/s, that the concentrations at the high- and low-pressure sides of the plate are 3.0 and 0.64 kg/m3 (kilogram of hydrogen per cubic meter of palladium), and that steady-state conditions have been attained.

Answers: 1
Another question on Engineering

Engineering, 04.07.2019 18:10
Compute the pressure drop of 30°c air flowing with a mean velocity of 8 m/s in a circular sheet-metal duct 300 mm in diameter and 15 m long. use a friction factor, f 0.02, and pair = 1.1644 kg/m a. 37.26 pa b. 25.27 pa n c. 29.34 pa d. 30.52 pa
Answers: 1

Engineering, 04.07.2019 18:20
Atank with constant volume contains 2.27 kg of a mixture of water phases (liquid-vapor). in the initial state the temperature and the quality are 127 °c and 0.6, respectively. the mixture is heated until the temperature of 160 oc is reached. illustrate the process in a t-v diagram. then, determine (1) the mass of the vapor in kg at the initial state, (2) the final pressure in kpa.
Answers: 3

Engineering, 04.07.2019 18:20
A2-m rigid tank initially contains saturated water vapor at 100 kpa. the tank is connected to a supply line through a valve. steam is flowing in the supply line at 600 kpa and 300 c. the valve is opened, and steam is allowed to enter the tank until the pressure in the tank reaches the line pressure, at which point the valve is closed. a thermometer placed in the tank indicates that the temperature at the final state is 200°c. determine (a) the mass of steam that has entered the tank (b) the amount of heat transfer.
Answers: 3

Engineering, 04.07.2019 19:10
10 kg of co2 is initially contained at 400 kpa and 300 k. the gas constant for carbon dioxide is 189 j/lkg k) and has a specific heat ratio, k, of 1.289. isentropic expansion then occurs until the pressure is 200 kpa. a) determine the initial volume of co2 in m. b) determine the final temperature in k. c) determine the work done by the system during the expansion kl.
Answers: 2
You know the right answer?
The purification of hydrogen gas is possible by diffusion through a thin palladium sheet. Calculate...
Questions

Mathematics, 18.08.2019 20:00


History, 18.08.2019 20:00


Mathematics, 18.08.2019 20:00

History, 18.08.2019 20:00

Mathematics, 18.08.2019 20:00






Mathematics, 18.08.2019 20:00

Mathematics, 18.08.2019 20:00

Mathematics, 18.08.2019 20:00

English, 18.08.2019 20:00

Mathematics, 18.08.2019 20:00


Mathematics, 18.08.2019 20:00

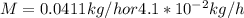
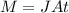
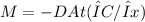
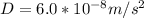
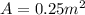
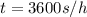
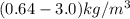
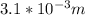
![M=-(6.0*10^{-8} m/s^{2})(0.25 m^{2})(3600 s/h)[(0.64-3.0kg/m^{3})(3.1*10^{-3}m)]](/tpl/images/0560/5193/8d176.png)


