
Engineering, 30.03.2020 16:59 paulitaaustin
The reverse water-gas shift (RWGS) reaction is an equimolar reaction between CO2 and H2 to form CO and H2O. Assume CO2 associatively adsorbs to the surface, while H2 dissociatively adsorbs. These adsorption steps are followed by reversible formation of formate (COOH*) and slow dissociation of formate into gaseous CO and adsorbed OH. The adsorbed OH is then removed as gaseous H2O via a hydrogenation step.
a) Using the details of the mechanism listed above, write out the elementary steps for the RWGS reaction.
b) Derive a rate law for the RWGS reaction consistent with the above assumptions and mechanism from (i).
c) Under what conditions is the RWGS reaction first order in CO2?

Answers: 3
Another question on Engineering

Engineering, 04.07.2019 18:10
Compute the pressure drop of 30°c air flowing with a mean velocity of 8 m/s in a circular sheet-metal duct 300 mm in diameter and 15 m long. use a friction factor, f 0.02, and pair = 1.1644 kg/m a. 37.26 pa b. 25.27 pa n c. 29.34 pa d. 30.52 pa
Answers: 1

Engineering, 04.07.2019 18:20
Atank with constant volume contains 2.27 kg of a mixture of water phases (liquid-vapor). in the initial state the temperature and the quality are 127 °c and 0.6, respectively. the mixture is heated until the temperature of 160 oc is reached. illustrate the process in a t-v diagram. then, determine (1) the mass of the vapor in kg at the initial state, (2) the final pressure in kpa.
Answers: 3

Engineering, 04.07.2019 19:10
What is a monomer? how do they form a ploymer from the view point of chemical bonding?
Answers: 1

Engineering, 04.07.2019 19:20
In the winter, in order to keep the classroom steadily at 68 f before 10 pm, heating with an average rate of 42,000 btu/hr is provided. assume the outdoor temperature maintains at 32°f, determine the electrical power (kw) required to (a) operate a reversible heat pump (b) operate a real heat pump with a cop (7) of 4.5 and (c) operate an electrical-resistance heater.
Answers: 3
You know the right answer?
The reverse water-gas shift (RWGS) reaction is an equimolar reaction between CO2 and H2 to form CO a...
Questions



History, 20.02.2020 06:40

Chemistry, 20.02.2020 06:40



Mathematics, 20.02.2020 06:40




English, 20.02.2020 06:40


History, 20.02.2020 06:40


Computers and Technology, 20.02.2020 06:40





Chemistry, 20.02.2020 06:41

![Rate = \frac{k_{1}k_{4} }{k_{3}+ 2k_{4} } [H_{2} ]](/tpl/images/0570/4445/89073.png)
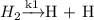 (Fast process)
(Fast process)![\[ CO_{2} + H\mathrel{\mathop{\rightleftarrows}^{\mathrm{k2}}_{\mathrm{k3}}}COOH \]](/tpl/images/0570/4445/0ee99.png) (Fast Process)
(Fast Process)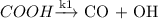 (Slow process)
(Slow process)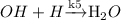 (Fast process)
(Fast process)![\frac{d[COOH]}{dt} = 0](/tpl/images/0570/4445/953ae.png)
![k_{2} [CO_{2} ][H] = k_{3} [COOH] k_{4} [COOH]\\](/tpl/images/0570/4445/d3edf.png)
![[COOH] = \frac{k_{2} [CO_{2} ][H]}{k_{3}k_{4} } \\](/tpl/images/0570/4445/2668d.png)
![\frac{d[H]}{dt} = 0](/tpl/images/0570/4445/8807a.png)
![k_{1}[H_{2}] = k_{2}[CO_{2} [H]+k_{5} [ OH][H]](/tpl/images/0570/4445/93949.png)
![[H]= \frac{k_{1}[H_{2}] }{k_{5}[OH] +k_{2}[CO_{2}]}\\](/tpl/images/0570/4445/c726a.png)
![\frac{d[OH]}{dt} = 0](/tpl/images/0570/4445/d1756.png)
![k_{4} [COOH] = k_{5} [OH][H]\\\k[OH] = \frac{k_{4} [COOH]}{k_{5} H}\\](/tpl/images/0570/4445/833a9.png)
![Rate = k_{4} [COOH]\\](/tpl/images/0570/4445/d9edf.png)
![Rate = k_{4} \frac{k_{2} [CO_{2} ][H]}{k_{3}k_{4} }\\Rate = k_{4} \frac{k_{2}[CO_{2}]\frac{k_{1}[H_{2}] }{k_{5}[OH] +k_{2}[CO_{2}]} }{k_{3}k_{4}}\\Rate = k_{4} \frac{k_{2}[CO_{2}]\frac{k_{1}[H_{2}] }{k_{5}\frac{k_{4}COOH }{k_{5}H } +k_{2}[CO_{2}]} }{k_{3}k_{4}}](/tpl/images/0570/4445/8876a.png)


