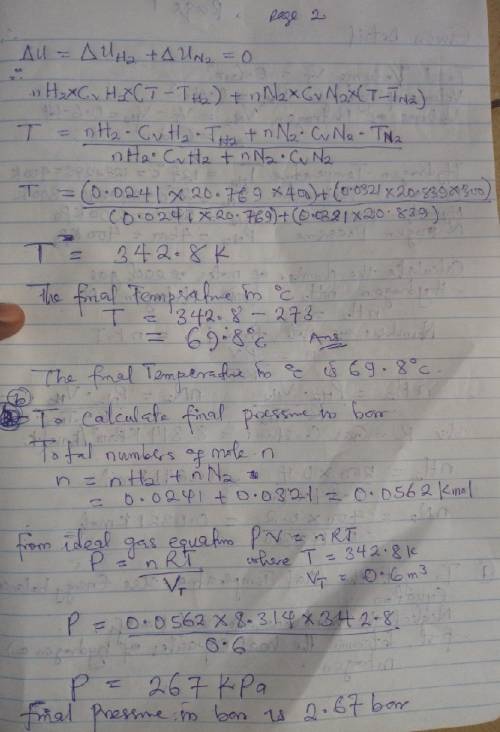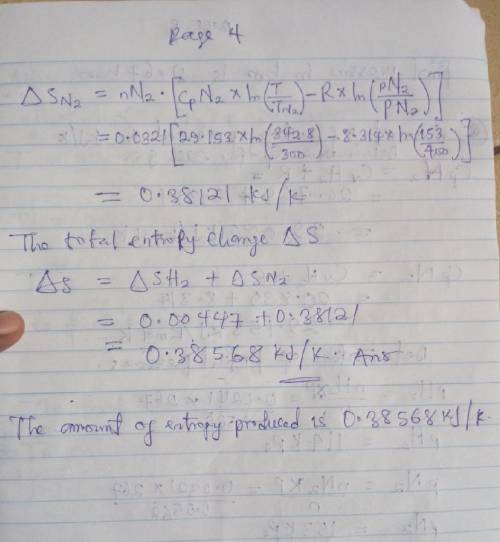Engineering, 30.03.2020 20:35 angel41vgg
An insulated tank having a total volume of 0.6 m3 is divided into two compartments. Initially one compartment contains 0.4 m3 of hydrogen at 127 oC, 2 bar and the other contains nitrogen at 27 oC, 4 bar. The gases are allowed to mix until an equilibrium state is attained. Assume the ideal gas model with constant specific heats, determine
a. The final temperature, in oC.
b. The final pressure, in bar.
c. The amount of entropy produced, in KJ/K.

Answers: 3
Another question on Engineering

Engineering, 04.07.2019 16:10
An electrical motor raises a 50kg load at a construct velencity .calculate the power of the motor, if it takes 40sec to raise the load through a height of 24m(take g =9.8n/g)
Answers: 2

Engineering, 04.07.2019 18:10
Asingle-geared blanking press has a stroke of 200 mm and a rated capacity of 320 kn. a cam driven ram is assumed to be capable of delivering the full press load at constant force during the last 15 percent of a constant-velocity stroke. the camshaft has an average speed of 90 rev/min and is geared to the flywheel shaft at a 6: 1 ratio. the total work done is to include an allowance of 16 percent for friction a) estimate the maximum energy fluctuation b) find the rim weight for an effective diameter of 1.2 m and a coefficient of speed fluctuation of 0.10
Answers: 1

Engineering, 04.07.2019 18:20
Find the kinematic pressure of 160kpa. for air, r-287 j/ kg k. and hair al viscosity of air at a temperature of 50°c and an absolute (10 points) (b) find the dynamic viscosity of air at 110 °c. sutherland constant for air is 111k
Answers: 3

Engineering, 04.07.2019 19:10
What is a monomer? how do they form a ploymer from the view point of chemical bonding?
Answers: 1
You know the right answer?
An insulated tank having a total volume of 0.6 m3 is divided into two compartments. Initially one co...
Questions




Chemistry, 23.01.2020 03:31

History, 23.01.2020 03:31

Mathematics, 23.01.2020 03:31

Mathematics, 23.01.2020 03:31

Social Studies, 23.01.2020 03:31

Mathematics, 23.01.2020 03:31

Mathematics, 23.01.2020 03:31



Mathematics, 23.01.2020 03:31

History, 23.01.2020 03:31

Mathematics, 23.01.2020 03:31



Social Studies, 23.01.2020 03:31

History, 23.01.2020 03:31

Mathematics, 23.01.2020 03:31