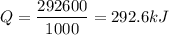Engineering, 30.03.2020 21:25 coolkitty35
Find the amount of energy (Q) required to raise the temperature of the water in kilo joules (KJ).
Given mass of water m = 2kg = 2000g.
Temperature difference ΔΤ = T2 – T1 = 60 °C – 25 °C = 35 °C .
Specific heat of water = 4.18 J/g* °C .
Therefore, Q =
kilo joules.

Answers: 3
Another question on Engineering

Engineering, 04.07.2019 18:10
The mass flow rate of the fluid remains constant in all steady flow process. a)- true b)- false
Answers: 1

Engineering, 04.07.2019 18:10
Steel is coated with a thin layer of ceramic to protect against corrosion. what do you expect to happen to the coating when the temperature of the steel is increased significantly? explain.
Answers: 1

Engineering, 04.07.2019 18:10
Burgers vector is generally parallel to the dislocation line. a)-true b)-false
Answers: 2

Engineering, 04.07.2019 19:10
Estimate the change in specific internal energy au and specific enthalpy h from inlet to outlet for ethylene glycol (a liquid) flowing through each of the following devices: (a) a heat exchanger where the glycol temperature increases from 20 °c to 80 °c; (b) a pump operating at about 25 °c and increasing the glycol pressure from 100 kpa to 8 mpa.
Answers: 2
You know the right answer?
Find the amount of energy (Q) required to raise the temperature of the water in kilo joules (KJ).
Questions




History, 20.11.2019 01:31


Chemistry, 20.11.2019 01:31



Mathematics, 20.11.2019 01:31


Chemistry, 20.11.2019 01:31

Mathematics, 20.11.2019 01:31

Mathematics, 20.11.2019 01:31


History, 20.11.2019 01:31

Biology, 20.11.2019 01:31


English, 20.11.2019 01:31

Mathematics, 20.11.2019 01:31