
Engineering, 07.04.2020 03:16 mimi5937
Applying the Entropy Balance: Closed Systems Five kg of carbon dioxide (CO2) gas undergoes a process in a well-insulated piston–cylinder assembly from 2 bar, 280 K to 20 bar, 520 K. If the carbon dioxide behaves as an ideal gas, determine the amount of entropy produced, in kJ/K, assuming (a) constant specific heats with c= = 0.939 kJ/kg · K. (b) variable specific heats. Compare the results of parts (a) and (b).

Answers: 2
Another question on Engineering

Engineering, 04.07.2019 18:10
True or false (explain) (110)[111] is a slip system in bcc metals . the {111} family in fcc contains 8 planes. resolved shear stress (rss) in single crystals is just related to the applied stress. critical resolved shear stress (crss) in single crystal metals is direct proportional to the number of defects in the structure
Answers: 2

Engineering, 04.07.2019 18:10
Which from the following instrument is commonly used to detect the high pitch butzing sound in bearings? [clo4] a)-digital ultrasonic meter b)-infrared camera c)-spectroscopic d)-vibrometer
Answers: 2

Engineering, 04.07.2019 18:10
Journeyman training is usually related (clo2) a)-to specific tasks b)-to cost analysis of maintenance task c)-to control process to ensure quality d)-to installation of machinery
Answers: 2

Engineering, 04.07.2019 19:10
What is a monomer? how do they form a ploymer from the view point of chemical bonding?
Answers: 1
You know the right answer?
Applying the Entropy Balance: Closed Systems Five kg of carbon dioxide (CO2) gas undergoes a process...
Questions



English, 20.10.2019 05:10


Geography, 20.10.2019 05:10

Mathematics, 20.10.2019 05:10

Health, 20.10.2019 05:10


Mathematics, 20.10.2019 05:10








Mathematics, 20.10.2019 05:10

Biology, 20.10.2019 05:10

Business, 20.10.2019 05:10

Social Studies, 20.10.2019 05:10

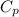 = 0.939 kJ/kg.K , m = 5 kg , T₂ = 520 K , T₁ = 280
= 0.939 kJ/kg.K , m = 5 kg , T₂ = 520 K , T₁ = 280![m[c_p ln(\frac{T_2}{T_1}) - Rln(\frac{P_2}{P_1})]](/tpl/images/0585/8806/1d12a.png)
![5[(0.939) ln(\frac{520}{280}) - (\frac{8.314}{44.01})ln(\frac{20}{2})]](/tpl/images/0585/8806/340b4.png)
![m[\frac{s^0(T_2) - s^0(T_1)}{M} - Rln(\frac{P_2}{P_1})]](/tpl/images/0585/8806/932df.png)
![5[\frac{236.575 - 211.376}{44.01} - (\frac{8.314}{44.01})ln(\frac{20}{2})]](/tpl/images/0585/8806/335ff.png)


