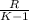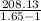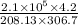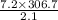Engineering, 07.04.2020 02:48 kenneditomejia
Argon contained in a closed, rigid tank, initially at 33.7°C, 2.1 bar, and a volume of 4.2 m3, is heated to a final pressure of 7.2 bar. Assuming the ideal gas model with k = 1.65 for the argon, determine the heat transfer, in kJ.

Answers: 2
Another question on Engineering

Engineering, 03.07.2019 14:10
If the thermal strain developed in polyimide film during deposition is given as 0.0044. assume room temperature is kept at 17.3 c, and thermal coefficient of expansion for the film and the substrate are 54 x 10^-6c^-1 and 3.3 x 10^-6c^-1respectively. calculate the deposition temperature.
Answers: 3

Engineering, 03.07.2019 15:10
Two flowing streams of argon gas are adiabatically mixed to form a single flow/stream. one stream is 1.5 kg/s at 400 kpa and 200 c while the second stream is 2kg/s at 500 kpa and 100 ? . it is stated that the exit state of the mixed single flow of argon gas is 150 c and 300 kpa. assuming there is no work output or input during the mixing process, does this process violate either the first or the second law or both? explain and state all your assumptions.
Answers: 1

Engineering, 04.07.2019 18:10
The higher the astm grain-size number, the coarser the grain is. a)-true b)-false
Answers: 3

Engineering, 04.07.2019 18:10
A-mn has a cubic structure with a0 0.8931 nm and a density of 7.47 g/cm3. b-mn has a different cubic structure, with a0 0.6326 nm and a density of 7.26 g/cm3. the atomic weight of manganese is 54.938 g/mol and the atomic radius is 0.112 nm. determine the percent volume change that would occur if a-mn transforms to b-mn.
Answers: 2
You know the right answer?
Argon contained in a closed, rigid tank, initially at 33.7°C, 2.1 bar, and a volume of 4.2 m3, is he...
Questions



Chemistry, 30.10.2021 22:40

Mathematics, 30.10.2021 22:40



Mathematics, 30.10.2021 22:40




Health, 30.10.2021 22:40



Mathematics, 30.10.2021 22:50

Chemistry, 30.10.2021 22:50




Computers and Technology, 30.10.2021 22:50

Social Studies, 30.10.2021 22:50