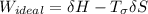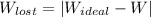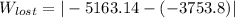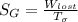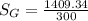Engineering, 11.04.2020 02:51 claudia3776
A steady flow adiabatic turbine accepts gas at conditions T1, P1 and discharges at conditions T2 and P2. Assuming ideal gas, determine (per mole of gas) W, Wideal, Wlost and SG for the following. Take Tσ = 300 Κ, Τ1 = 500 Κ, P1 = 6 bar, Τ2 = 371 Κ, P2 = 1.2 bar, and Cp/R = 7/2.
Chemical Engineering (thermodynamics) Please answer as soon as possible.

Answers: 2
Another question on Engineering

Engineering, 03.07.2019 14:10
The y form of iron is known as: a) ferrite b) cementite c) perlite d) austenite
Answers: 3

Engineering, 04.07.2019 18:10
The filament of an incandescent lamp has a temperature of 2000k. calculate the fraction of radiation emitted in the visible light band if the filament is approximated as blackbody
Answers: 2

Engineering, 04.07.2019 18:10
The higher the astm grain size number, the finer the gran is. a)-true b)-false
Answers: 2

Engineering, 04.07.2019 18:10
Which of the following components of a pid controlled accumulates the error over time and responds to system error after the error has been accumulated? a)- proportional b)- derivative c)- integral d)- on/off.
Answers: 2
You know the right answer?
A steady flow adiabatic turbine accepts gas at conditions T1, P1 and discharges at conditions T2 and...
Questions

Mathematics, 28.09.2021 21:30

Social Studies, 28.09.2021 21:30


Chemistry, 28.09.2021 21:30

Mathematics, 28.09.2021 21:30

English, 28.09.2021 21:30

Geography, 28.09.2021 21:30


Mathematics, 28.09.2021 21:30


Mathematics, 28.09.2021 21:30

Mathematics, 28.09.2021 21:30

Mathematics, 28.09.2021 21:30


Mathematics, 28.09.2021 21:30

Mathematics, 28.09.2021 21:30


Chemistry, 28.09.2021 21:30

Arts, 28.09.2021 21:30

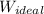 = - 5163.14 J
= - 5163.14 J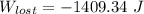
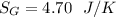
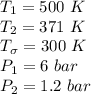
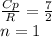
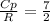
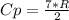
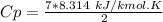
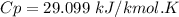
![n[Cp In \frac{T_2}{T_1} - R In \frac{P_2}{P_1}]](/tpl/images/0594/9077/c65c6.png)
![1*[29.099 In(\frac{371}{500}) - 8.314 In (\frac{1.2}{6})]](/tpl/images/0594/9077/c221b.png)