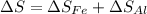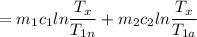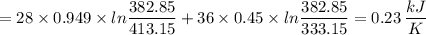Engineering, 18.06.2020 04:57 justinbailey96
An aluminum block weighing 28 kg initially at 140°C is brought into contact with a block of iron weighing 36 kg at 60°C in an insulated enclosure. Determine the final equilibrium temperature and the total entropy change for this process. The specific heat of aluminum at 400 K is cp = 0.949 kJ/kg·K. The specific heat of iron at room temperature is cp = 0.45 kJ/kg·K.

Answers: 3
Another question on Engineering

Engineering, 03.07.2019 15:10
Heat is added to a piston-cylinder device filled with 2 kg of air to raise its temperature 400 c from an initial temperature of t1 27 cand pressure of pi 1 mpa. the process is isobaric process. find a)-the final pressure p2 b)-the heat transfer to the air.
Answers: 1

Engineering, 04.07.2019 18:10
During a steady flow process, the change of energy with respect to time is zero. a)- true b)- false
Answers: 2

Engineering, 04.07.2019 18:10
Carbon dioxide gas expands isotherm a turbine from 1 mpa, 500 k at 200 kpa. assuming the ideal gas model and neglecting the kinetic and potential energies, determine the change in entropy, heat transfer and work for each kilogram of co2.
Answers: 2

Engineering, 04.07.2019 18:10
Shafts are machine elements that are used to a) carry axial loads b) direct shear loads c) transmit power d) rotate at constant speed e) none of the above circular and square shafts subjected to the same torque under the same circum behave a) the same way b) almost the same way
Answers: 2
You know the right answer?
An aluminum block weighing 28 kg initially at 140°C is brought into contact with a block of iron wei...
Questions

Social Studies, 22.12.2021 20:50

SAT, 22.12.2021 20:50




SAT, 22.12.2021 20:50

Computers and Technology, 22.12.2021 20:50




SAT, 22.12.2021 21:00

Mathematics, 22.12.2021 21:00


Computers and Technology, 22.12.2021 21:00



English, 22.12.2021 21:00

SAT, 22.12.2021 21:00