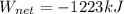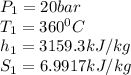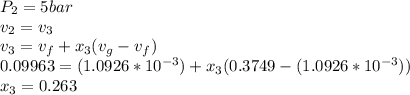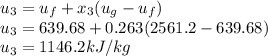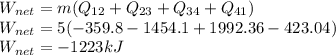Engineering, 24.06.2020 02:01 kace04
A piston-cylinder assembly contains 5kg of water that undergoes a series of processes to form a thermodynamic cycle. Process 1à 2: Constant pressure cooling from p1=20bar and T1=360°C to saturated vapor Process 2à 3: Constant volume cooling to p3=5 bar Process 3à 4: Constant pressure heating Process 4à 1: Polytropic process following Pv =constant back to the initial state Kinetic and potential energy effects are negligible. Calculate the net work for the cycle in kJ.

Answers: 2
Another question on Engineering

Engineering, 04.07.2019 18:10
The higher the astm grain-size number, the coarser the grain is. a)-true b)-false
Answers: 3

Engineering, 04.07.2019 18:20
Inspection for bearing condition will include: (clo4) a)-color b)-smell c)-size d)-none of the above
Answers: 1

Engineering, 04.07.2019 18:20
Atank with constant volume contains 2.27 kg of a mixture of water phases (liquid-vapor). in the initial state the temperature and the quality are 127 °c and 0.6, respectively. the mixture is heated until the temperature of 160 oc is reached. illustrate the process in a t-v diagram. then, determine (1) the mass of the vapor in kg at the initial state, (2) the final pressure in kpa.
Answers: 3

Engineering, 04.07.2019 19:10
Air inially occupying a volume of 1 m2 at 100 kpa, 27 c undergoes three internally reversible processes in series. process 1-2 compression to 500 kpa during which pv constant process 2-3 adiabatic expanslon to 100 kpa process 3-1: constant-pressure expansion to 100 kpa (a) calculate the change of entropy for each of the three processes. (b) calculate the heat and work involved in each process. (c) is this cycle a power cycle or refrigeration cycle?
Answers: 3
You know the right answer?
A piston-cylinder assembly contains 5kg of water that undergoes a series of processes to form a ther...
Questions




Chemistry, 28.01.2020 07:31

Mathematics, 28.01.2020 07:31





Mathematics, 28.01.2020 07:31


Mathematics, 28.01.2020 07:31

History, 28.01.2020 07:31

Chemistry, 28.01.2020 07:31


Biology, 28.01.2020 07:31



Chemistry, 28.01.2020 07:31