
Engineering, 24.07.2020 01:01 lanapope
Steam undergoes an isentropic compression in an insulatedpiston–cylinder assembly from an initial state where T1= 160°C, p1= 1 bar to a final state where the pressure p2= 30bar. Determinethe final temperature, in °C, and the work, in kJ per kg of steam

Answers: 2
Another question on Engineering

Engineering, 04.07.2019 18:10
Apipe with an outside diameter of 15 cm is exposed to an ambient air and surrounding temperature of -20°c. the pipe has an outer surface temperature of 65°c and an emissivity of 0.85. if the rate of heat loss from the pipe surface is 0.95 kw per meter of length, the external convective heat transfer coefficient (h) is: (a) 12.5 w/m"k (b) 18.6 w/mk (c) 23.7 w/mk (d) 27.9 w/mk (e) 33.5 w/mk
Answers: 1

Engineering, 04.07.2019 18:10
Shafts are machine elements that are used to a) carry axial loads b) direct shear loads c) transmit power d) rotate at constant speed e) none of the above circular and square shafts subjected to the same torque under the same circum behave a) the same way b) almost the same way
Answers: 2

Engineering, 04.07.2019 18:10
Journeyman training is usually related (clo2) a)-to specific tasks b)-to cost analysis of maintenance task c)-to control process to ensure quality d)-to installation of machinery
Answers: 2

Engineering, 04.07.2019 18:20
Ahe-xe mixture containing a 0.75 mole fraction of helium is used for cooling electronics in an avionics application. at a temperature of 300 k and atmospheric pressure, calculate the mass fraction of helium and the mass density, molar concentration and molecular weight of the mixture. if the cooling capacity is 10 l, what is the mass of the coolant?
Answers: 3
You know the right answer?
Steam undergoes an isentropic compression in an insulatedpiston–cylinder assembly from an initial st...
Questions

Advanced Placement (AP), 16.10.2020 09:01


Computers and Technology, 16.10.2020 09:01



History, 16.10.2020 09:01


Mathematics, 16.10.2020 09:01


Mathematics, 16.10.2020 09:01



English, 16.10.2020 09:01


History, 16.10.2020 09:01



Mathematics, 16.10.2020 09:01

Mathematics, 16.10.2020 09:01

Biology, 16.10.2020 09:01

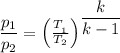
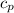 ×(T₂ - T₁)
×(T₂ - T₁) 

