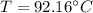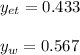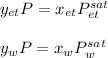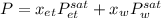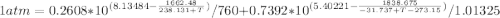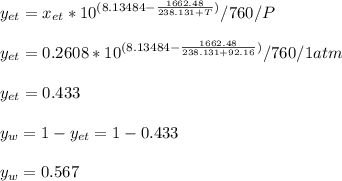Engineering, 02.09.2020 01:01 jordanrose98
Using Raoult’s law, estimate the boiling temperature and mole fractions in the vapor phase that is in equilibrium with a liquid having 0.2608 mol fraction of ethanol, in a mixture ethanol/water at P = 1 atm. If possible, solve non-numerically.

Answers: 3
Another question on Engineering

Engineering, 03.07.2019 15:10
Apiston-cylinder with a volume of 0.25 m3 holds 1 kg of air (r 0.287 k/kgk) at a temperature of 100 c. heat transfer to the cylinder causes an isothermal expansion of the piston until the volume triples. how much heat is added to the piston-cylinder?
Answers: 3

Engineering, 03.07.2019 15:10
Ahouse has the following electrical appliance usage (1) single 40w lamp used for 4 hours per day (2) single 60w fan used for 12 hours per day (3) single 200w refrigerator that runs 24 hours per day with compressor run 12 hours and off 12 hours find the solar power inverter size in watt with correction factor of 1.25.
Answers: 1

Engineering, 04.07.2019 19:20
The process in which the system pressure remain constant is called a)-isobaric b)-isochoric c)-isolated d)-isothermal
Answers: 3

Engineering, 04.07.2019 19:20
Brief discuss how the presence of dislocations in crystal structures can be an advantage and a disadvantage to engineer and designers.
Answers: 3
You know the right answer?
Using Raoult’s law, estimate the boiling temperature and mole fractions in the vapor phase that is i...
Questions

Mathematics, 13.07.2021 01:00


Chemistry, 13.07.2021 01:00



Mathematics, 13.07.2021 01:00

English, 13.07.2021 01:00

Mathematics, 13.07.2021 01:00

English, 13.07.2021 01:00

Mathematics, 13.07.2021 01:00


Geography, 13.07.2021 01:00

Arts, 13.07.2021 01:00







Mathematics, 13.07.2021 01:00