
Engineering, 12.10.2020 15:01 BreBreDoeCCx
Steam at 20 bars is in the saturated vapor state (call this state 1) and contained in a pistoncylinderdevice with a volume of 0.03 m3. Assume the steam is cooled at constantvolume (i. e. the piston is held fixed in place) until the temperature reaches 200 C (callthis state 2). Then the steam is expanded isothermally until its volume is three times theinitial value (state 3).
Required:
a. Determine the pressures at state 2 and 3. ans. 15.5 bar, ~10 bar
b. Determine the change in specific internal energy, u, for each of the two processes.
-389 kJ/kg, 410 kJ/kg
c. Make qualitatively correct sketches of the processes on a T-v plot.

Answers: 2
Another question on Engineering

Engineering, 04.07.2019 18:10
Draw the engineering stress-strain curve for (a) bcc; (b) fcc metals and mark important points.
Answers: 1

Engineering, 04.07.2019 18:20
Most leaks in reciprocating air compressors can be detected and minimized by: (clo4) a)-detecting leakage areas using ultrasonic acoustic detector. b)-tightening joints and connections c)-replacing faulty equipment d)-all of the given options
Answers: 2

Engineering, 04.07.2019 18:20
Select any two (2) areas of applications of chain-drive. (clo4) a)-permanent lubrication necessary b)-hydraulic forklift truck operation c)-rigging and heavy moving materials d)-relatively high maintenance costs e)-costlier than belt drives
Answers: 2

Engineering, 04.07.2019 19:10
Plan an experiment to measure the surface tension of a liquid similar to water. if necessary, review the ncfmf video surface tension for ideas. which method would be most suitable for use in an undergraduate laboratory? what experimental precision could be expected?
Answers: 2
You know the right answer?
Steam at 20 bars is in the saturated vapor state (call this state 1) and contained in a pistoncylind...
Questions



Mathematics, 28.04.2021 08:50


Mathematics, 28.04.2021 08:50


Social Studies, 28.04.2021 08:50

Advanced Placement (AP), 28.04.2021 08:50

English, 28.04.2021 08:50

Business, 28.04.2021 08:50

Business, 28.04.2021 08:50



Mathematics, 28.04.2021 08:50



Mathematics, 28.04.2021 08:50

Chemistry, 28.04.2021 08:50


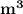
 = 0.0996
= 0.0996 

 , the saturated pressure at state 2 i.e. P₂ = 15.5 bar
, the saturated pressure at state 2 i.e. P₂ = 15.5 bar 
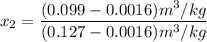

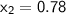
 , also
, also 






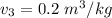
 ,
,  , therefore, the phase is in a superheated vapour state.
, therefore, the phase is in a superheated vapour state.
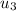 at the pressure of 10 bar = 2622.3 kJ/kg
at the pressure of 10 bar = 2622.3 kJ/kg




