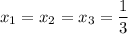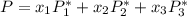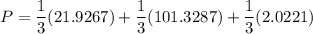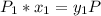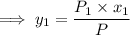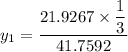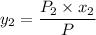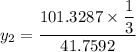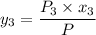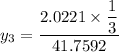Engineering, 09.11.2020 16:20 mia2286
Equal moles of pure liquid 1-Butanol, Benzene, and Phenol form an ideal solution system at 353K. Determine Yi for each component at vapor-liquid equilibrium (VLE) ofthe mixtureat 353K.

Answers: 2
Another question on Engineering

Engineering, 04.07.2019 18:10
What difference(s) did you notice using a pneumatic circuit over hydraulic circuit.explain why the pneumatic piston stumbles when it hits an obstacle.
Answers: 2

Engineering, 04.07.2019 18:10
The thermal expansion or contraction of a given metal is a function of the f a)-density b)-initial temperature c)- temperature difference d)- linear coefficient of thermal expansion e)- final temperature f)- original length
Answers: 2

Engineering, 04.07.2019 18:10
Items are similar to the free issue items, but their access is limited. (clo5) a)-bin stock items free issue b)-bin stock controlled issue c)-critical or insurance spares d)-rebuildable spares e)-consumables
Answers: 1

Engineering, 04.07.2019 18:20
Wiy doeres rere okhn a pump whon working betwon the same pressure range?
Answers: 2
You know the right answer?
Equal moles of pure liquid 1-Butanol, Benzene, and Phenol form an ideal solution system at 353K. Det...
Questions

Biology, 19.09.2019 19:30

Biology, 19.09.2019 19:30

Computers and Technology, 19.09.2019 19:30


Mathematics, 19.09.2019 19:30

Mathematics, 19.09.2019 19:30

Mathematics, 19.09.2019 19:30

Mathematics, 19.09.2019 19:30






Mathematics, 19.09.2019 19:30

Biology, 19.09.2019 19:30





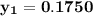
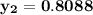
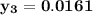
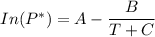
![P_1 ^* = exp \bigg [15.314 - \dfrac{3212.43}{80+182.739} \bigg ]](/tpl/images/0880/0793/b66c2.png)
![P_1 ^* = exp \bigg [15.314 - \dfrac{3212.43}{262.739} \bigg ]](/tpl/images/0880/0793/20f3b.png)
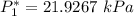
![P_2^* = exp \bigg [13.7819 - \dfrac{2726.81}{80+217.572} \bigg ]](/tpl/images/0880/0793/8425c.png)
![P_2^* = exp \bigg [13.7819 - \dfrac{2726.81}{297.572} \bigg ]](/tpl/images/0880/0793/a0ef9.png)

![P_3^* = exp \bigg [14.4387 - \dfrac{3507.80}{80+175.400} \bigg ]](/tpl/images/0880/0793/b6ec1.png)
![P_3^* = exp \bigg [14.4387 - \dfrac{3507.80}{255.4} \bigg ]](/tpl/images/0880/0793/b9d14.png)
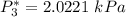
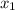 = 1/3,
= 1/3, 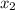 = 1/3,
= 1/3, 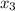 = 1/3,
= 1/3,