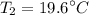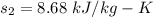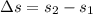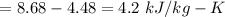Engineering, 25.11.2020 14:00 zyairemitchell44
A rigid, insulated vessel is divided into two equal-volume compartments connected by a valve. Initially, one compartment con tains 1 m3 of water at 20°C, x = 50%, and the other is evacuated. The valve is opened and the water is allowed to fill the entire volume. For the water, determine the final temperature, in °C, and the amount of entropy produced, in kJ/K.

Answers: 1
Another question on Engineering

Engineering, 04.07.2019 03:10
What precautions should you take to prevent injuries when dealing with heavy loads?
Answers: 1

Engineering, 04.07.2019 18:10
An air conditioning system consist of a 5 cm diameter pipe, operating at a pressure of 200 kpa. the air initially enters the pipe at 15°c with a velocity of 20 m/s and relative humidity of 80%. if the heat supply throughout the process is 960 w, determine the relative humidity and the temperature at the outlet
Answers: 3

Engineering, 04.07.2019 18:20
Agas mixture consists of 8 kmol of h2 and 2 kmol of n2. determine the mass of each gas and the apparent gas constant of the mixture.
Answers: 3

Engineering, 04.07.2019 18:20
Ahe-xe mixture containing a 0.75 mole fraction of helium is used for cooling electronics in an avionics application. at a temperature of 300 k and atmospheric pressure, calculate the mass fraction of helium and the mass density, molar concentration and molecular weight of the mixture. if the cooling capacity is 10 l, what is the mass of the coolant?
Answers: 3
You know the right answer?
A rigid, insulated vessel is divided into two equal-volume compartments connected by a valve. Initia...
Questions

Spanish, 01.07.2019 13:30



History, 01.07.2019 13:30


Mathematics, 01.07.2019 13:30


Biology, 01.07.2019 13:30

Biology, 01.07.2019 13:30

English, 01.07.2019 13:30

Mathematics, 01.07.2019 13:30

English, 01.07.2019 13:30

Mathematics, 01.07.2019 13:30

Spanish, 01.07.2019 13:30

Biology, 01.07.2019 13:30

Mathematics, 01.07.2019 13:30

History, 01.07.2019 13:30


Chemistry, 01.07.2019 13:30

History, 01.07.2019 13:30

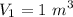
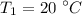
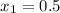
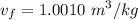
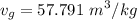
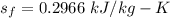
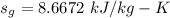
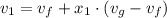
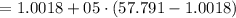
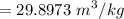
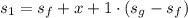
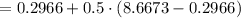
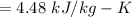
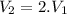
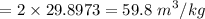
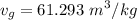 , we can get final temperature and specific entropy corresponding to value of
, we can get final temperature and specific entropy corresponding to value of 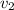 :
: