
Engineering, 21.12.2020 23:20 ella3714
The van der Waals equation is a modification of the ideal gas equation. What two factors does this equation account for? A. (1) Real gas molecules exert forces on each other. (2) Gas molecules have energy. B. (1) Real gas molecules exert ionic forces on each other. (2) Gas molecules have energy C. (1) Real gas molecules exert forces on each other. (2) Gas molecules have volume. D. (1) Real gas molecules exert ionic forces on each other. (2) Gas molecules have volume. E. None of the above have BOTH of the two factors accurately stated HINT: Check Van der Waals equation.

Answers: 2
Another question on Engineering

Engineering, 04.07.2019 12:10
On a average work day more than work place firs are reorted
Answers: 1

Engineering, 04.07.2019 19:10
The short distance from the objective lens to the object causes problems at high magnification. which of the following is the most serious? a. cleaning the object surface b. positioning the object c. reflection from the object surface. d. illumination of the object
Answers: 1

Engineering, 04.07.2019 19:10
The proportional limit is always greater than the yield strength for a material. a)-trune b)- false
Answers: 3

Engineering, 06.07.2019 03:20
What is the strain energy stored in a solid uniformly circular shaft of length 3.0 m and diameter 120 mm when subject to pure torsion if the maximum shear stress is 40 mpa? g-80gpa
Answers: 1
You know the right answer?
The van der Waals equation is a modification of the ideal gas equation. What two factors does this e...
Questions





History, 10.12.2019 00:31


History, 10.12.2019 00:31

History, 10.12.2019 00:31



English, 10.12.2019 00:31

English, 10.12.2019 00:31


Mathematics, 10.12.2019 00:31

Mathematics, 10.12.2019 00:31


Mathematics, 10.12.2019 00:31



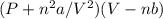 =nRT
=nRT

