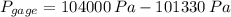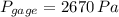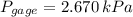Engineering, 12.02.2021 01:00 izzyisawesome5232
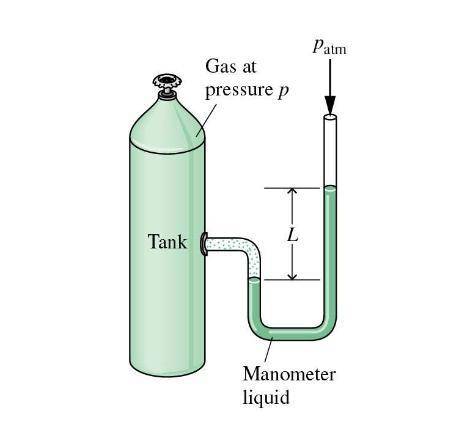
As shown in the figure below, a monometer is attached to a tank of gas in which the pressure is 104.0 kPa. The manometer liquid is mercury, with a density of 13.59 g/cm 3 . If g = 9.81 m/s 2 and the atmospheric pressure is 101.33 kPa, calculate (a) the difference in mercury levels in the manometer, in cm, and (b) the gage pressure of the gas, in kPa.

Answers: 3
Another question on Engineering

Engineering, 04.07.2019 18:10
The mass flow rate of the fluid remains constant in all steady flow process. a)- true b)- false
Answers: 1

Engineering, 04.07.2019 18:10
Condition monitoring is a major component of. (clo4) a)- predictive maintenance. b)-preventive maintenance c)-proactive maintenance d)-reactive maintenance.
Answers: 1

Engineering, 04.07.2019 18:10
Which one from below is not one of the reasons of planning failures? (clo3) a)-planner is careless. b-planner spend less time in the field but more time on the desk c)-planner is not qualified d)-planner does not have sufficient time to properly plan
Answers: 3

Engineering, 04.07.2019 18:20
Atank with constant volume contains 2.27 kg of a mixture of water phases (liquid-vapor). in the initial state the temperature and the quality are 127 °c and 0.6, respectively. the mixture is heated until the temperature of 160 oc is reached. illustrate the process in a t-v diagram. then, determine (1) the mass of the vapor in kg at the initial state, (2) the final pressure in kpa.
Answers: 3
You know the right answer?
As shown in the figure below, a monometer is attached to a tank of gas in which the pressure is 104....
Questions



Mathematics, 30.12.2020 09:20


Mathematics, 30.12.2020 09:20

History, 30.12.2020 09:20

Chemistry, 30.12.2020 09:20

Mathematics, 30.12.2020 09:20

Chemistry, 30.12.2020 09:20



Chemistry, 30.12.2020 09:20

Mathematics, 30.12.2020 09:20

Computers and Technology, 30.12.2020 09:20

Mathematics, 30.12.2020 09:20

Mathematics, 30.12.2020 09:30

Social Studies, 30.12.2020 09:30

Mathematics, 30.12.2020 09:30

Computers and Technology, 30.12.2020 09:30

Computers and Technology, 30.12.2020 09:30

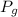 ) and atmospheric pressure (
) and atmospheric pressure (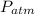 ), both measured in pascals. A kilopascal equals 1000 pascals and 1 meter equals 100 centimeters. That is:
), both measured in pascals. A kilopascal equals 1000 pascals and 1 meter equals 100 centimeters. That is: 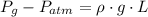 (1)
(1)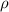 - Density of mercury, measured in kilograms per cubic meter.
- Density of mercury, measured in kilograms per cubic meter.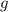 - Gravitational acceleration, measured in meters per square second.
- Gravitational acceleration, measured in meters per square second.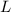 - Difference in mercury levels, measured in meters.
- Difference in mercury levels, measured in meters. 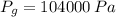 ,
, 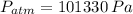 ,
, 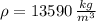 and
and 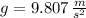 , the difference in mercury levels in the manometer is:
, the difference in mercury levels in the manometer is: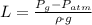
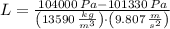
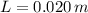
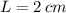
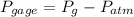 (2)
(2)