
Engineering, 23.03.2021 07:10 sammuelanderson1539
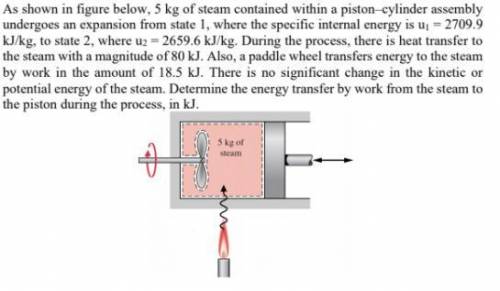
5 kg of steam contained within a piston–cylinder assembly undergoes an expansion from state 1, where the specific internal energy is u1 = 2709.9 kJ/kg, to state 2, where u2 = 2659.6 kJ/kg. During the process, there is heat transfer to the steam with a magnitude of 80 kJ. Also, a paddle wheel transfers energy to the steam by work in the amount of 18.5 kJ. There is no significant change in the kinetic or potential energy of the steam. Determine the energy transfer by work from the steam to the piston during the process, in kJ.

Answers: 2
Another question on Engineering

Engineering, 04.07.2019 18:10
Draw the engineering stress-strain curve for (a) bcc; (b) fcc metals and mark important points.
Answers: 1

Engineering, 04.07.2019 18:20
Aquick transition of the operating speed of a shaft from its critical speed will whirl amplitude. (a) increase (b) limit (c) not affect (d) zero
Answers: 2

Engineering, 04.07.2019 18:20
Air is compressed isentropically from an initial state of 300 k and 101 kpa to a final temperature of 1000 k. determine the final pressure using the following approaches: (a) approximate analysis (using properties at the average temperature) (b) exact analysis
Answers: 1

Engineering, 04.07.2019 19:10
The air in an automobile tire with a volume of 0.015 m3 is at 32°c and 140 kpa gage. determine the amount of air that must be added to raise the pressure to the recommended value of 206 kpa gage. assume the atmospheric pressure to be 128 kpa and the temperature and the volume to remain constant.[r-0.287 kj/kgk]
Answers: 3
You know the right answer?
5 kg of steam contained within a piston–cylinder assembly undergoes an expansion from state 1, where...
Questions

History, 02.09.2021 04:20



Mathematics, 02.09.2021 04:20

English, 02.09.2021 04:20

Mathematics, 02.09.2021 04:20

Mathematics, 02.09.2021 04:20

History, 02.09.2021 04:20


Spanish, 02.09.2021 04:20



Mathematics, 02.09.2021 04:20


Mathematics, 02.09.2021 04:20


Mathematics, 02.09.2021 04:20

English, 02.09.2021 04:20

Mathematics, 02.09.2021 04:20




