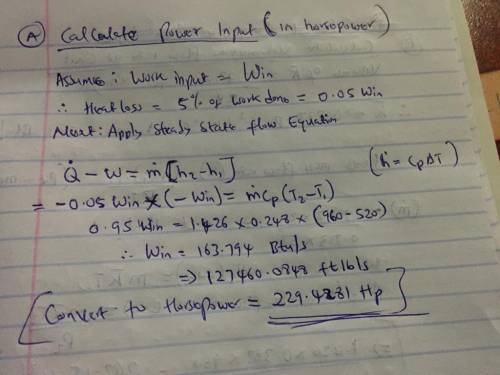Engineering, 02.04.2021 01:50 Athenax
Nitrogen gas is compressed at steady state from a pressure of 14.2 psi and a temperature 60o F to a pressure of 120 psi and a temperature of 500o F. The gas enters a compressor with a volumetric flow rate of 1200 ft3 /min. The magnitude of the heat transfer rate from the compressor to its surroundings is 5% of the compressor power input. Using the ideal gas model (with variable specific heats) and neglecting kinetic and potential energy effects, determine (a) The compressor power input (in horsepower). (b) The volumetric flow rate at the exit (in ft3 /min).

Answers: 1
Another question on Engineering

Engineering, 03.07.2019 15:10
Two flowing streams of argon gas are adiabatically mixed to form a single flow/stream. one stream is 1.5 kg/s at 400 kpa and 200 c while the second stream is 2kg/s at 500 kpa and 100 ? . it is stated that the exit state of the mixed single flow of argon gas is 150 c and 300 kpa. assuming there is no work output or input during the mixing process, does this process violate either the first or the second law or both? explain and state all your assumptions.
Answers: 1

Engineering, 04.07.2019 03:10
What precautions should you take to prevent injuries when dealing with heavy loads?
Answers: 1

Engineering, 04.07.2019 18:20
Most leaks in reciprocating air compressors can be detected and minimized by: (clo4) a)-detecting leakage areas using ultrasonic acoustic detector. b)-tightening joints and connections c)-replacing faulty equipment d)-all of the given options
Answers: 2

Engineering, 04.07.2019 19:10
Analyze the factors that influence the choice between the following pairs of processes to make the products indicated: i) sand casting versus die casting of an electric-motor housing ii) thread rolling versus machining of a bolt for high-strength application. (co3/c5)
Answers: 3
You know the right answer?
Nitrogen gas is compressed at steady state from a pressure of 14.2 psi and a temperature 60o F to a...
Questions

Mathematics, 28.08.2019 15:30


Mathematics, 28.08.2019 15:30





Biology, 28.08.2019 15:30

Biology, 28.08.2019 15:30


Biology, 28.08.2019 15:30


Mathematics, 28.08.2019 15:30

Physics, 28.08.2019 15:30

Geography, 28.08.2019 15:30


Biology, 28.08.2019 15:30

Spanish, 28.08.2019 15:30