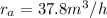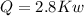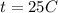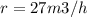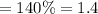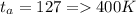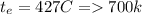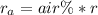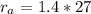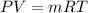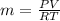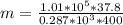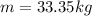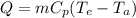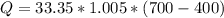Engineering, 14.06.2021 22:10 spiritedawayoy6378
Methane gas (CH4) at 25oC, 1 atm, and a volumetric flow rate of 27m3/h enters a furnace operating at steady-state. The methane burns completely with 140% of theoretical air that enters at 127oC, 1 atm. Products of combustion exit at 427oC, 1 atm. Determine:
(a) the volumetric flow rate of the air, in m3/h.
(b) the rate of heat transfer from the furnace, in kJ/h.

Answers: 3
Another question on Engineering

Engineering, 04.07.2019 12:10
On a average work day more than work place firs are reorted
Answers: 1

Engineering, 04.07.2019 18:10
Assuming compressible flow of air and that the measurements are done at flagstaff a pitot static tube that gives the difference of total and static pressure measures 0.35 m of mercury. what is the velocity of air? assume the temperature to be 300k. (submit your excel or matlab calculation sheet)
Answers: 1

Engineering, 04.07.2019 18:10
True or false (explain) (110)[111] is a slip system in bcc metals . the {111} family in fcc contains 8 planes. resolved shear stress (rss) in single crystals is just related to the applied stress. critical resolved shear stress (crss) in single crystal metals is direct proportional to the number of defects in the structure
Answers: 2

Engineering, 04.07.2019 19:10
Air inially occupying a volume of 1 m2 at 100 kpa, 27 c undergoes three internally reversible processes in series. process 1-2 compression to 500 kpa during which pv constant process 2-3 adiabatic expanslon to 100 kpa process 3-1: constant-pressure expansion to 100 kpa (a) calculate the change of entropy for each of the three processes. (b) calculate the heat and work involved in each process. (c) is this cycle a power cycle or refrigeration cycle?
Answers: 3
You know the right answer?
Methane gas (CH4) at 25oC, 1 atm, and a volumetric flow rate of 27m3/h enters a furnace operating at...
Questions

Mathematics, 15.04.2021 21:20



Social Studies, 15.04.2021 21:20





Mathematics, 15.04.2021 21:20


Business, 15.04.2021 21:20

Mathematics, 15.04.2021 21:20




Mathematics, 15.04.2021 21:20




English, 15.04.2021 21:20