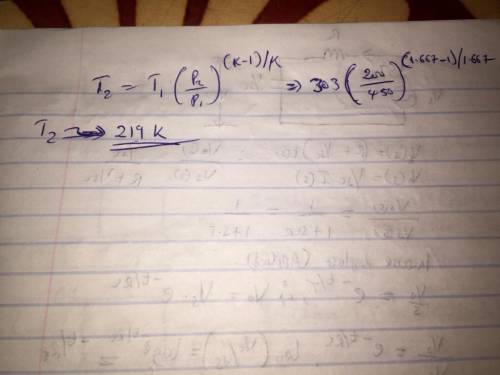Engineering, 02.07.2021 02:50 AliMe52
A well insulated rigid tank contains 4 kg of argon gas at 450 kPa and 30 C. A valve is opened, allowing the argon to escape until the tank pressure drops to 200 kPa. Assuming that the argon remaining in the tank experiences a reversible adiabatic process, find the final mass of argon in the tank. Since you don't have argon gas tables, assume cp, cv, k as needed at some appropriate temperature(s).

Answers: 3
Another question on Engineering

Engineering, 04.07.2019 16:10
An electrical motor raises a 50kg load at a construct velencity .calculate the power of the motor, if it takes 40sec to raise the load through a height of 24m(take g =9.8n/g)
Answers: 2

Engineering, 04.07.2019 18:10
Which of the following ziegler nichols tuning methods the response of the controller to a step input should exhibit an s-shaped curve? a)-open loop mode b)-closed loop mode c)-both modes (open & closed) d)-none of the modes (open & closed)
Answers: 3

Engineering, 04.07.2019 18:10
Calculate the bore of a cylinder that has a stroke of 18 inches and an extension time of 6 seconds at a flow rate of 4 gal/min.
Answers: 3

Engineering, 04.07.2019 18:20
Derive the correction factor formula for conical nozzle i=-(1+ cosa) and calculate the nozzle angle correction factor for a nozzle whose divergence hal-fangle is 13 (hint: assume that all the mass flow originates at the apex of the cone.
Answers: 3
You know the right answer?
A well insulated rigid tank contains 4 kg of argon gas at 450 kPa and 30 C. A valve is opened, allow...
Questions

Social Studies, 25.11.2021 09:50

English, 25.11.2021 09:50

History, 25.11.2021 09:50

Computers and Technology, 25.11.2021 09:50





Mathematics, 25.11.2021 09:50

Physics, 25.11.2021 09:50

Chemistry, 25.11.2021 09:50

Mathematics, 25.11.2021 09:50


History, 25.11.2021 09:50




English, 25.11.2021 09:50