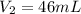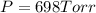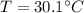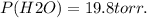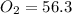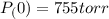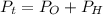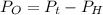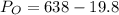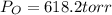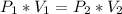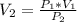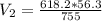Engineering, 20.07.2021 01:00 Wolfgirl2032
Calculate the pressure of dry O2 if the total pressure of O2 generated over water is measured to be 698 Torr and the temperature is 30.1 oC. P(H2O) = 19.8 torr. If the volume of the O2 sample in the question above was 56.3 ml, what volume would the dry O2 occupy at 755 torr (assume the temp was unchanged).

Answers: 1
Another question on Engineering

Engineering, 04.07.2019 08:10
Which of the following is an easy way to remember the modified “x” tire rotation? a. nondrive wheels straight, cross the drive wheels b. drive wheels straight, cross the nondrive wheels c. drive wheels crossed, nondrive wheels straight d. drive wheels crossed, nondrive wheels crossed
Answers: 1

Engineering, 04.07.2019 18:10
Steel is coated with a thin layer of ceramic to protect against corrosion. what do you expect to happen to the coating when the temperature of the steel is increased significantly? explain.
Answers: 1

Engineering, 04.07.2019 18:10
Assuming compressible flow of air and that the measurements are done at flagstaff a pitot static tube that gives the difference of total and static pressure measures 0.35 m of mercury. what is the velocity of air? assume the temperature to be 300k. (submit your excel or matlab calculation sheet)
Answers: 1

Engineering, 04.07.2019 18:10
Journeyman training is usually related (clo2) a)-to specific tasks b)-to cost analysis of maintenance task c)-to control process to ensure quality d)-to installation of machinery
Answers: 2
You know the right answer?
Calculate the pressure of dry O2 if the total pressure of O2 generated over water is measured to be...
Questions


History, 18.02.2021 01:00


Mathematics, 18.02.2021 01:00




Mathematics, 18.02.2021 01:00





English, 18.02.2021 01:00

History, 18.02.2021 01:00





History, 18.02.2021 01:00