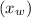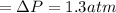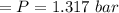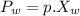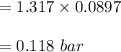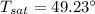n-Octane (C8H18) is burned with 60 percent excess air with 15 percent of the carbon in the fuel forming carbon monoxide. Calculate the mole fractions of the products and the dew-point temperature of the water vapor in the products when the products are at 1.3 atm pressure. Use data from the tables. The mole fraction of CO2 is .0678the mole fraction of CO is .012the mole fraction of H2O is .0897the mole fraction of O2 is .0808and the mole fraction of N2 is .7498The dew-point temperature of the water vapor is °C.

Answers: 3
Another question on English

English, 21.06.2019 16:20
Which type of conflict does della face in o. henry’s “the gift of the magi”? a. character versus society b. character versus machine c. character versus character d. character versus nature
Answers: 2

English, 21.06.2019 20:00
Which of the following statements is a claim of definition?
Answers: 1

English, 22.06.2019 00:30
Which sentence uses all its nominative case pronouns correctly? a we live on the same street b them and rachel are good skaters. c the redskins fans in the family are fred and me d have joanne and him played tennis together before?
Answers: 1

English, 22.06.2019 02:00
Respond for free points because i don't have a question anymore.
Answers: 1
You know the right answer?
n-Octane (C8H18) is burned with 60 percent excess air with 15 percent of the carbon in the fuel form...
Questions

Chemistry, 28.09.2021 16:10


History, 28.09.2021 16:10





English, 28.09.2021 16:10



Mathematics, 28.09.2021 16:10


Mathematics, 28.09.2021 16:10


Mathematics, 28.09.2021 16:20

English, 28.09.2021 16:20




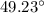 "
"