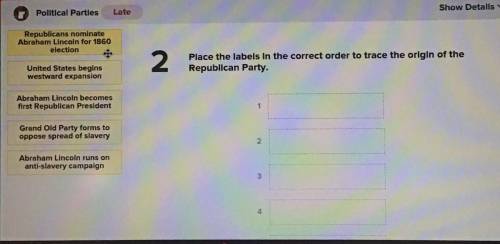
Helppp me pls
...

Answers: 2
Another question on Law

Law, 03.07.2019 15:10
Asubject in a clinical research trial experiences a serious, unanticipated adverse drug experience. how should the investigator proceed, with respect to the irb, after the discovery of the adverse event occurrence? a. do not report the adverse drug experience to the irb since it is a common adverse experience. b. report the adverse drug experience to the irb only if there are several other occurrences. c. report the adverse drug experience as part of the continuing review report. d. report the adverse drug experience in a timely manner, in keeping with the irb's policies and procedures, using the forms or the mechanism provided by the irb.
Answers: 2



Law, 06.07.2019 11:10
Vop hearing iowa 2 tec violation discharge date expired tho can they send me to jail?
Answers: 3
You know the right answer?
Questions

Mathematics, 15.12.2021 21:50






History, 15.12.2021 21:50



Mathematics, 15.12.2021 21:50



Mathematics, 15.12.2021 21:50

History, 15.12.2021 21:50

Social Studies, 15.12.2021 21:50

Mathematics, 15.12.2021 21:50


Chemistry, 15.12.2021 21:50

Physics, 15.12.2021 21:50