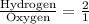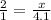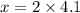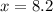Mathematics, 01.07.2019 02:30 titalili0204
The ratio of hydrogen to oxygen atoms in water molecules is 2 to 1. if the beaker of water contained 4.1 moles of oxygen atoms, how many moles of hydrogen atoms did it contain? (note: a mole is a unit commonly used in chemistry. you don't need to know this to solve the problem, but in case you are interested, 1 mole=6.02 times 10^23 atoms) plz !

Answers: 1
Another question on Mathematics

Mathematics, 21.06.2019 16:00
Asquare parking lot has 6,400 square meters what is the length in meters
Answers: 1

Mathematics, 21.06.2019 17:20
Consider the expression below. 9 + 4(x + 2) – 3.1 select the term that best describes "3" in the given expression. o a. coefficient variable exponent constant
Answers: 2


You know the right answer?
The ratio of hydrogen to oxygen atoms in water molecules is 2 to 1. if the beaker of water contained...
Questions

Spanish, 11.09.2020 19:01

Mathematics, 11.09.2020 19:01

Mathematics, 11.09.2020 19:01

Mathematics, 11.09.2020 19:01

Social Studies, 11.09.2020 19:01

Mathematics, 11.09.2020 19:01

Mathematics, 11.09.2020 19:01

Mathematics, 11.09.2020 19:01

Mathematics, 11.09.2020 19:01

Mathematics, 11.09.2020 19:01

Mathematics, 11.09.2020 19:01

Mathematics, 11.09.2020 19:01

Mathematics, 11.09.2020 19:01

Mathematics, 11.09.2020 19:01

English, 11.09.2020 19:01

Mathematics, 11.09.2020 19:01

Mathematics, 11.09.2020 19:01

Mathematics, 11.09.2020 19:01

English, 11.09.2020 19:01

Mathematics, 11.09.2020 19:01