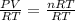Mathematics, 21.08.2019 05:00 jordan2875
In chemistry, n molecules of an ideal gas have pressure (p), volume (v), and temperature (t) that stand in the following relation, where r is a constant. solve the equation for n, the number of molecules.


Answers: 2
Another question on Mathematics

Mathematics, 21.06.2019 13:30
Factorize: x^2+8x+10 i just solved it but i don't know if the process is correct
Answers: 3

Mathematics, 21.06.2019 20:30
Daryl factors the polynomial p(x)=x3+x2−26x+24 to rewrite it as p(x)=(x+6)(x−4)(x−1). which equations must be true? there may be more than one correct answer. select all correct answers. p(1)=0 p(−4)=0 p(−1)=0 p(6)=0 p(4)=0 p(−6)=0
Answers: 1

Mathematics, 21.06.2019 21:30
Carl's candies has determined that a candy bar measuring 3 inches long has a z-score of +1 and a candy bar measuring 3.75 inches long has a z-score of +2. what is the standard deviation of the length of candy bars produced at carl's candies?
Answers: 1

You know the right answer?
In chemistry, n molecules of an ideal gas have pressure (p), volume (v), and temperature (t) that st...
Questions

Mathematics, 30.06.2019 16:30

Mathematics, 30.06.2019 16:30

Mathematics, 30.06.2019 16:30


Mathematics, 30.06.2019 16:30




English, 30.06.2019 16:30


Mathematics, 30.06.2019 16:30




History, 30.06.2019 16:30



Mathematics, 30.06.2019 16:30

Mathematics, 30.06.2019 16:30


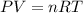 ......[1] where R is a constant.
......[1] where R is a constant.