
Mathematics, 01.08.2019 05:10 kactus
The standard cell potential (e°) of a voltaic cell constructed using the cell reaction below is 0.76 v: zn (s) + 2h+ (aq) → zn2+ (aq) + h2 (g) with ph2 = 1.0 atm and [zn2+] = 1.0 m, the cell potential is 0.52 v. the concentration of h+ in the cathode compartment is m.

Answers: 3
Another question on Mathematics

Mathematics, 21.06.2019 15:00
Find the balance at the end of 4 years if 1000 is deposited at the rate of
Answers: 2

Mathematics, 21.06.2019 18:10
Jordan has $5.37, which he is using to buy ingredients to make salsa. he is buying one red pepper for $1.29 and three pounds of tomatoes. if jordan has exactly the right amount of money he needs, what is the price per pound of the tomatoes? choose the correct equation to represent this real-world problem. solve the equation and verify the reasonableness of your answer. a pound of tomatoes costs .
Answers: 1

Mathematics, 21.06.2019 20:30
Jose is going to use a random number generator 500500 times. each time he uses it, he will get a 1, 2, 3,1,2,3, or 44.
Answers: 1

Mathematics, 21.06.2019 22:30
What fraction is equivalent to 0.46464646··· a. 46⁄999 b. 46⁄100 c. 46⁄99 d. 23⁄50
Answers: 1
You know the right answer?
The standard cell potential (e°) of a voltaic cell constructed using the cell reaction below is 0.76...
Questions

Mathematics, 07.01.2021 22:20


History, 07.01.2021 22:20



Mathematics, 07.01.2021 22:20

Mathematics, 07.01.2021 22:20



Mathematics, 07.01.2021 22:20

Mathematics, 07.01.2021 22:20


Mathematics, 07.01.2021 22:20


English, 07.01.2021 22:20


Mathematics, 07.01.2021 22:20



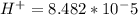 M
M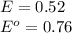
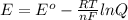
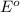 is the standard cell potential
is the standard cell potential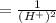
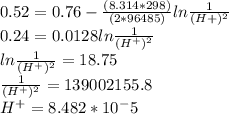 M
M

