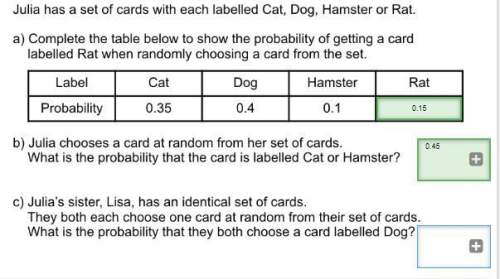
Mathematics, 03.08.2019 03:10 russboys3
Asecond important result is that electrons will fill the lowest energy states available. this would seem to indicate that every electron in an atom should be in the n=1 state. this is not the case, because of pauli's exclusion principle. the exclusion principle says that no two electrons can occupy the same state. a state is completely characterized by the four numbers n, l, ml, and ms, where ms is the spin of the electron. an important question is, how many states are possible for a given set of quantum numbers? for instance, n=1 means that l=0 with ml=0 are the only possible values for those variables. thus, there are two possible states: (1, 0, 0, 1/2) and (1, 0, 0, −1/2). how many states are possible for n=2?

Answers: 1
Another question on Mathematics

Mathematics, 21.06.2019 13:30
I'm being volume of cylinder with 13 in. radius and 30 in height answer choices in pic
Answers: 2

Mathematics, 21.06.2019 18:00
Look at arnold's attempt to solve the equation for b: 3b = 12 b = 3 · 12 b = 36 describe the mistake that arnold made.
Answers: 2

Mathematics, 21.06.2019 22:30
The pair of coordinates that do not represent the point( 5,150’) is a. (5,-210) b. (5,210) c. (-5,330) d. (-5,-30)
Answers: 1

Mathematics, 21.06.2019 22:30
Acredit union pays 5% annual interest, compounded daily, on savings deposits. find the value after one year of $500 deposited in this account. $525.64 $25.64 $20.40 $520.40
Answers: 2
You know the right answer?
Asecond important result is that electrons will fill the lowest energy states available. this would...
Questions



Spanish, 15.01.2021 01:10


Mathematics, 15.01.2021 01:10

Mathematics, 15.01.2021 01:10

Mathematics, 15.01.2021 01:10

English, 15.01.2021 01:10


Mathematics, 15.01.2021 01:10



Health, 15.01.2021 01:10




History, 15.01.2021 01:10


Mathematics, 15.01.2021 01:10

History, 15.01.2021 01:10