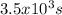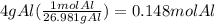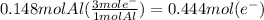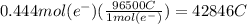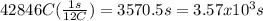Mathematics, 09.10.2019 16:10 erykp17
How many seconds are required to produce 4.00 g of aluminum metal from the electrolysis of molten alcl3 with an electrical current of 12.0 a? how many seconds are required to produce 4.00 g of aluminum metal from the electrolysis of molten alcl3 with an electrical current of 12.0 a? 3.57 × 103 1.19 × 103 9.00 2.90 × 105 27.0

Answers: 1
Another question on Mathematics

Mathematics, 21.06.2019 18:00
What power can you write to represent the volume of the cube shown? write the power as an expression with a base and an exponent and then find the volume of the cube
Answers: 3

Mathematics, 21.06.2019 20:00
Choose the linear inequality that describes the graph. the gray area represents the shaded region. a: y ≤ –4x – 2 b: y > –4x – 2 c: y ≥ –4x – 2 d: y < 4x – 2
Answers: 2

Mathematics, 21.06.2019 20:30
Elizabeth claims that the fourth root of 2 can be expressed as 2^m since (2^m)^n = 2. find the values of m and n for the case where elizabeth's claim is true.
Answers: 3

Mathematics, 21.06.2019 21:00
Mr.zimmerman invested $25,000 in an account that draws 1.4 interest, compouneded annually. what is the total value of the account after 15 years
Answers: 1
You know the right answer?
How many seconds are required to produce 4.00 g of aluminum metal from the electrolysis of molten al...
Questions





Mathematics, 28.09.2021 20:40

English, 28.09.2021 20:40

Mathematics, 28.09.2021 20:40

Chemistry, 28.09.2021 20:40


Mathematics, 28.09.2021 20:40



Mathematics, 28.09.2021 20:40

Mathematics, 28.09.2021 20:40



Mathematics, 28.09.2021 20:40

Engineering, 28.09.2021 20:40

Mathematics, 28.09.2021 20:40

English, 28.09.2021 20:40