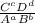Mathematics, 09.10.2019 23:00 lagster11
R--> 2 p. initially you start off with 0.5 m reactants and let the reaction run to equilibrium and calculate the equilibrium constant, k, value. what happens to that value if you run the reaction to equilibrium again at the same temperature but double the initial concentration of the reactants to 1.0 m?

Answers: 3
Another question on Mathematics

Mathematics, 21.06.2019 17:20
Which of these equations, when solved, gives a different value of x than the other three? a9.1 = -0.2x + 10 b10 = 9.1 + 0.2x c10 – 0.2x = 9.1 d9.1 – 10 = 0.2x
Answers: 1



Mathematics, 22.06.2019 05:30
To increase her number of students, the instructor put a coupon in the local paper offering to waive the one-time fee for new students. what is the value of the coupon?
Answers: 1
You know the right answer?
R--> 2 p. initially you start off with 0.5 m reactants and let the reaction run to equilibrium a...
Questions

Mathematics, 21.05.2020 03:02


Mathematics, 21.05.2020 03:02






Computers and Technology, 21.05.2020 03:02







English, 21.05.2020 03:02