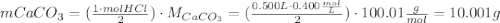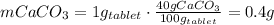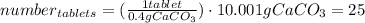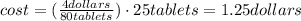Mathematics, 23.11.2019 02:31 davisdarby2
One container of tums® costs 4.00 dollars. each container has eighty 1.00 g tablets. assume each tums® is 40.0 percent caco₃ by mass. using only tums®, you are required to neutralize 0.500 l of 0.400 m hcl. how much does this cost? assume you are able to purchase individual tablets. express your answer in dollars.

Answers: 1
Another question on Mathematics


Mathematics, 21.06.2019 18:00
Which shows the correct solution of the equation 1/2a+2/3b=50, when b=30?
Answers: 1

Mathematics, 21.06.2019 18:00
List the sides of δrst in in ascending order (shortest to longest) if: m∠r =x+28°, m∠s = 2x+16°, and m∠t = x+12°
Answers: 1

Mathematics, 21.06.2019 18:30
Abus travels 36 miles in 45 minutes. enter the number of miles the bus travels in 60 minutes at this rate.
Answers: 2
You know the right answer?
One container of tums® costs 4.00 dollars. each container has eighty 1.00 g tablets. assume each tum...
Questions


World Languages, 30.12.2019 09:31



History, 30.12.2019 09:31






Mathematics, 30.12.2019 09:31


English, 30.12.2019 09:31

Physics, 30.12.2019 09:31

Biology, 30.12.2019 09:31

Computers and Technology, 30.12.2019 09:31


Business, 30.12.2019 09:31

Mathematics, 30.12.2019 09:31