
Mathematics, 23.11.2019 03:31 Frostbite9371
Consider the following time series data of sales per year: year sales 1995 22.2 1996 23.0 1997 24.4 1998 25.8 1999 27.6 enter these data in excel and run the following regression: sales = intercept + slope * year note that the year is the independent variable and sales is the dependent variable. a 95% confidence interval for the slope is:
a. (-3233.88, -2068.92)
b. (1.04, 1.68)
c. (0.80, 1.88)
d. (0.92,1.76)
e. (-3482.53,-1820.27)

Answers: 1
Another question on Mathematics

Mathematics, 21.06.2019 19:30
Finally, the arena decides to offer advertising space on the jerseys of the arena’s own amateur volley ball team. the arena wants the probability of being shortlisted to be 0.14. what is this as a percentage and a fraction? what is the probability of not being shortlisted? give your answer as a decimal. those shortlisted are entered into a final game of chance. there are six balls in a bag (2 blue balls, 2 green balls and 2 golden balls). to win, a company needs to take out two golden balls. the first ball is not replaced. what is the probability of any company winning advertising space on their volley ball team jerseys?
Answers: 3

Mathematics, 21.06.2019 21:30
Aye asap pls ! markin da brainiest too ! btw da step choices are all add, distribute, divide, n subtract
Answers: 2

Mathematics, 22.06.2019 00:20
❤️ (geometry) does the construction demonstrate how to copy an angle correctly using technology a) yes; the distance between points a and f was used to create circle h b) yes; the distance between points f and g was used to create circle h c)no; the distance between points a and f was used to create circle h d) no; the distance between points f and g was used to create circle h
Answers: 2

Mathematics, 22.06.2019 03:10
Which of the following statements are true? (select all that apply.) a quasi-static process is one in which the system is never far from being in equilibrium. when a system can go from state 1 to state 2 by several different processes, the amount of heat absorbed by the system will be the same for all processes. the internal energy of a given amount of an ideal gas depends only on its absolute temperature. when a system can go from state 1 to state 2 by several different processes, the work done on the system will be the same for all processes. when a system can go from state 1 to state 2 by several different processes, the change in the internal energy of the system will be the same for all processes. for any substance that expands when heated, its cp is greater than its cv.
Answers: 2
You know the right answer?
Consider the following time series data of sales per year: year sales 1995 22.2 1996 23.0 1997 24.4...
Questions

Mathematics, 22.12.2020 20:00

Mathematics, 22.12.2020 20:00

Mathematics, 22.12.2020 20:00

Chemistry, 22.12.2020 20:00

Social Studies, 22.12.2020 20:00

Mathematics, 22.12.2020 20:00


Spanish, 22.12.2020 20:00

Mathematics, 22.12.2020 20:10


Computers and Technology, 22.12.2020 20:10


Mathematics, 22.12.2020 20:10

Mathematics, 22.12.2020 20:10

Mathematics, 22.12.2020 20:10

Mathematics, 22.12.2020 20:10

Mathematics, 22.12.2020 20:10

Mathematics, 22.12.2020 20:10

Mathematics, 22.12.2020 20:10

Mathematics, 22.12.2020 20:10

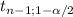 *(Sb/√n) is [1.04;1.68]
*(Sb/√n) is [1.04;1.68]

