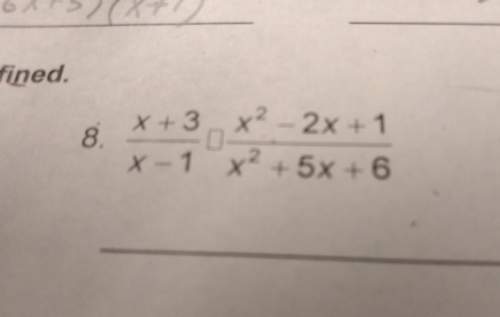How does the law of conservation of mass apply to this reaction:
c2h4 + 3o2 → 2h2o + 2co2 ?...

Mathematics, 03.12.2019 02:31 marchellamazz123
How does the law of conservation of mass apply to this reaction:
c2h4 + 3o2 → 2h2o + 2co2 ?
the equation needs to be balanced.
there are fewer oxygen atoms in the equation than hydrogen or carbon. only
the oxygen needs to be balanced.
there are equal numbers of hydrogen and carbon. each element needs to be
balanced.
the law of conservation of mass has already been applied. there is an equal
number of each element on both sides of the equation.

Answers: 1
Another question on Mathematics

Mathematics, 21.06.2019 13:00
How can we find the measure of an exterior angle if we know the measurement of an interior angle
Answers: 1

Mathematics, 21.06.2019 20:50
Find the equation of a line that is perpendicular to line g that contains (p, q). coordinate plane with line g that passes through the points negative 3 comma 6 and 0 comma 5 3x − y = 3p − q 3x + y = q − 3p x − y = p − q x + y = q − p
Answers: 1

Mathematics, 21.06.2019 22:10
Aadc is formed by reflecting aabc across line segment ac, as shown in the figure. if the length of ac is 4 units, the area of aadc is square units.
Answers: 3

Mathematics, 21.06.2019 23:30
The product of sin 30 degrees and sin 60 degrees is same as the product of
Answers: 1
You know the right answer?
Questions


History, 27.07.2019 05:30

Mathematics, 27.07.2019 05:30





Mathematics, 27.07.2019 05:30




History, 27.07.2019 05:30


History, 27.07.2019 05:30