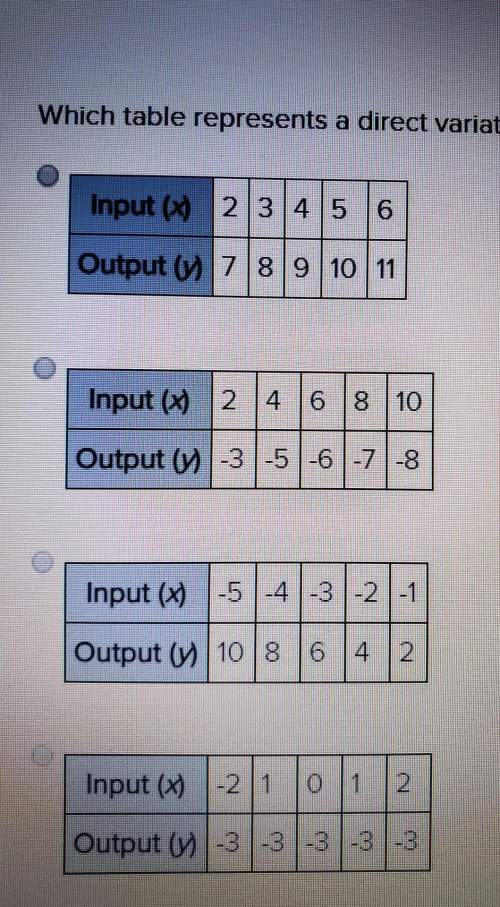
What is the quotient?
Negative 4 and one-third divided by negative three-fourths
N...

Mathematics, 18.02.2020 20:27 vaehcollier
What is the quotient?
Negative 4 and one-third divided by negative three-fourths
Negative 5 and StartFraction 7 over 9 EndFraction
Negative 5 and two-thirds
3 and one-fourth
5 and StartFraction 7 over 9 EndFraction

Answers: 2
Another question on Mathematics

Mathematics, 21.06.2019 19:40
Molly shared a spool of ribbon with 12 people. each person received 3 feet of ribbon. which equation can she use to find r, the number of feet of ribbon that her spool originally had?
Answers: 1

Mathematics, 22.06.2019 01:30
Asample of 200 rom computer chips was selected on each of 30 consecutive days, and the number of nonconforming chips on each day was as follows: the data has been given so that it can be copied into r as a vector. non.conforming = c(10, 15, 21, 19, 34, 16, 5, 24, 8, 21, 32, 14, 14, 19, 18, 20, 12, 23, 10, 19, 20, 18, 13, 26, 33, 14, 12, 21, 12, 27) #construct a p chart by using the following code. you will need to enter your values for pbar, lcl and ucl. pbar = lcl = ucl = plot(non.conforming/200, ylim = c(0,.5)) abline(h = pbar, lty = 2) abline(h = lcl, lty = 3) abline(h = ucl, lty = 3)
Answers: 3

Mathematics, 22.06.2019 02:00
Emily convinced her mom to buy a giant box of her favorite cereal. her mom doesn't think the box will fit on their shelf. the volume of the box is 1000 cm^3 . the base of the box is 25 cm by 10 cm
Answers: 1

Mathematics, 22.06.2019 03:10
Which of the following statements are true? (select all that apply.) a quasi-static process is one in which the system is never far from being in equilibrium. when a system can go from state 1 to state 2 by several different processes, the amount of heat absorbed by the system will be the same for all processes. the internal energy of a given amount of an ideal gas depends only on its absolute temperature. when a system can go from state 1 to state 2 by several different processes, the work done on the system will be the same for all processes. when a system can go from state 1 to state 2 by several different processes, the change in the internal energy of the system will be the same for all processes. for any substance that expands when heated, its cp is greater than its cv.
Answers: 2
You know the right answer?
Questions


Mathematics, 28.02.2020 19:17








Mathematics, 28.02.2020 19:17

History, 28.02.2020 19:17

Physics, 28.02.2020 19:17








Mathematics, 28.02.2020 19:17